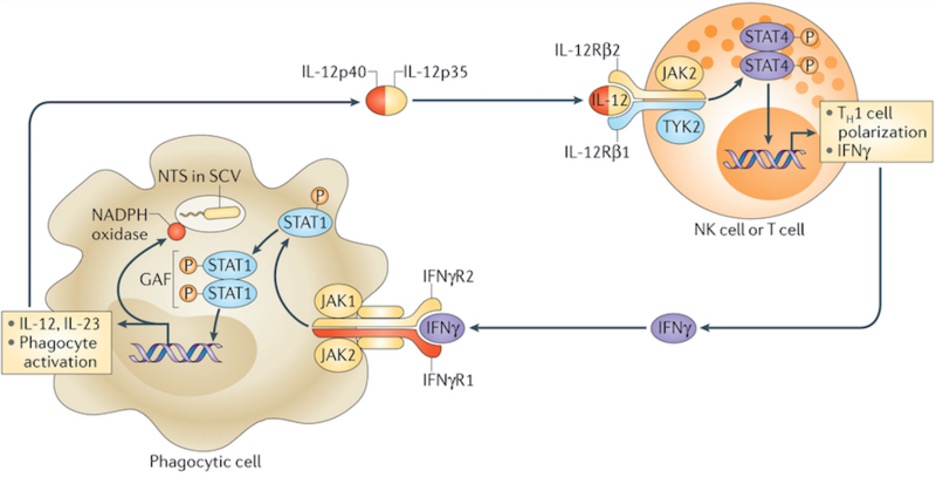
Figure 1: IFNγ (purple, bottom) is one kind of interferon, which are proteins that are key in activating cells vital to the immune response. Upon inhibition of its pathway, by mechanisms like those to be described, activation of those cells fails and immunosuppressive conditions result
Source: Wikimedia Commons
Interferons are a class of molecules involved in alerting the immune system of infection; they are implicated in general immune response quality as well as the amplification of antigen presentation to T cells, which regulate immune mechanisms and directly mediate killing of pathogen host cells (Le Page et al., 2000). Recent research suggests that inactivation of the type I interferon pathway, mediated by pathogen-infected cells, is necessary to drive the activity of myeloid-derived suppressor cells (MDSC) (Alicea-Torres et al., 2021). These findings have valuable implications because MDSCs are able to inhibit the functions of T cells, whose features are otherwise central in the antitumor response (Veglia et al., 2018). Researchers have already found a positive correlation between incidence of MDSC and cancer stage in many types, including bladder and thyroid cancers (Angell et al., 2016; Yang et al., 2017).
Generally, scientists have postulated that two types of white blood cells, monocytes and polymorphonuclear leukocytes (PMN), acquire immunosuppressive features in response to certain stimuli derived from the tumor microenvironment. However, recent research has indicated that only a fraction of PMN in cancer patients acquire said features; they account for 10% of PMN in the peripheral blood and 60% in tumor tissue (Condamine et al., 2016). In other words, there is a population of this cell type that does not become differentiated toward the immunosuppressive state; thus, there is the question of why abundant tumor-derived factors present in cancer patients do not affect all cells. Alicea-Torres et al. believe that, in addition to suppression-promoting tumor-derived factors that induce MDSCs, there are also negative signals that serve to regulate this differentiation and must be overcome for it to proceed (Alicea-Torres et al., 2021). The team noticed significant downregulation of the type I interferon (IFN1) pathway in MDSCs from prior data and decided to investigate. Essentially, this information was interesting because it could mean that downregulation of the IFN1 pathway contributes to the induction of MDSCs; in other words, the IFN1 pathway could belong to the group of regulatory mechanisms the team is proposing exists.
The IFNI pathway is a signal transduction cascade activated following release of interferon I. The protein binds to a receptor – consisting of two chains IFNAR1 and IFNAR – whose engagement triggers the activation of an enzyme that phosphorylates and activates the STAT1/STAT2 heterodimer. This protein, upon interacting with IRF9, translocates to the nucleus and activates transcription of IFN1-related genes (Platanias, 2005; Zitvogel et al., 2015). The genes stimulated by IFN1 are known to be involved in important processes including the improved survival of killer T lymphocytes as well as dendritic cell maturation, which is vital for successful antigen presentation to those T cells (Katlinski et al., 2017).
Alicea-Torres et al. found downregulation of the IFNAR1 chain specifically in MDSCs and consequently began exploring its relevance. Interestingly, research found that providing high amounts of IFNAR1 ligand in the absence of IFNAR1 itself entirely terminated suppressive MDSC activity, depicting IFN1 signaling as a negative regulator of MDSC differentiation. In a separate experiment, Alicea-Torres et al. also found that controlled downregulation of IFNAR1 expression in the mice being used for experimentation was not sufficient for increased MDSC activity; this was surprising as it is not what had been expected. However, the researchers explained this may have been because the mice already reached a maximum capacity for immunosuppressive activity that could not be built on (Alicea-Torres et al., 2021). Next, they sought to further understand the mechanisms involved in causing IFNAR1 downregulation as well as those resulting from it.
Existing research has yielded that decreased levels of IFNAR1 expression are attributed to its ubiquitination and degradation by two possible enzymes: D2 and p38 (Fuchs, 2013). Alicea-Torres et al. found that knocking out—or eliminating – p38 kinase was sufficient to avoid IFNAR1 downregulation, suggesting its significance in the degradation process. Concerning its role in contributing to MDSC activity, they were also able to conclude that IFNAR1 downregulation allowed for the increased expression of a variety of mediators that promote immunosuppressive conditions and activity (Alicea-Torres et al., 2021).
Building on the role of p38 kinase in IFNAR1 downregulation, experimental findings that p38 kinase knockout prevents negative regulation of IFNAR1 and favors an enhanced antitumor response are promising. They suggest that further exploring p38 kinase as it relates to the IFN1 pathway could lead to novel cancer treatments.
References
Alicea-Torres, K., Sanseviero, E., Gui, J. et al. (2021) Immune suppressive activity of myeloid-derived suppressor cells in cancer requires inactivation of the type I interferon pathway. Nat Commun 12, 1717
Angell, T. E. et al. (2016) Circulating myeloid-derived suppressor cells predict differentiated thyroid cancer diagnosis and extent. Thyroid 26, 381–389
Condamine, T. et al. (2016) Lectin-type oxidized LDL receptor-1 distinguishes population of human myeloid-derived suppressor cells in cancer patients. Sci. Immunol
Fuchs, S. Y. (2013) Hope and fear for interferon: the receptor-centric outlook on the future of interferon therapy. J. Interferon Cytokine Res. 33, 211–225
Katlinski, K. V. et al. (2017) Inactivation of interferon receptor promotes the establishment of immune privileged tumor microenvironment. Cancer Cell 31, 194–207
Le Page, C., Genin, P., Baines, M.G., Hiscott, J. (2000) Interferon activation and innate immunity. Rev Immunogenet. 2000;2(3):374-86.
Platanias, L. C. (2005) Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 5, 375–386
Veglia, F., Perego, M. & Gabrilovich, D. (2018) Myeloid-derived suppressor cells coming of age. Nat Immunol 19, 108–119
Yang, G. et al. (2017) Accumulation of myeloid-derived suppressor cells (MDSCs) induced by low levels of IL-6 correlates with poor prognosis in bladder cancer. Oncotarget 8, 38378–3838
Zitvogel, L., Galluzzi, L., Kepp, O., Smyth, M. J. & Kroemer, G. (2015) Type I interferons in anticancer immunity. Nat. Rev. Immunol. 15, 405–414
Related Posts
COVID-19 Pneumonia – A Different Illness Than Its Traditional Counterpart
Figure 1: This is an image of a child with...
Read MoreMETMAP: Mapping metastatic progression
Figure 1: The start of metastasis – the movement of...
Read MoreThe Role of Biometrics in Healthcare
This publication is in proud partnership with Project UNITY’s Catalyst...
Read MoreMatthew Lutchko