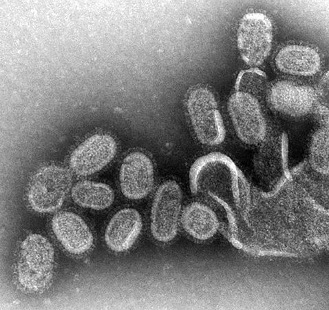
Figure 1: A negative stain transmission electron micrograph of Influenza virions
Source: Wikimedia Commons
Researchers at the University of Manchester have developed a new sugar-based virucidal medicine that is effective against a broad array of viruses (Jones et al., 2020). Cyclodextrins (also called CDs) are non-toxic cyclic glucose oligosaccharides produced as a result of starch (a polymer of glucose) breakdown in bacteria. CDs can be composed of 6 (called α-CD), 7 (β-CD), or 8 (γ-CD) glucose molecules [see Figure 2]. These cyclic molecules have been used to suspend and stabilize volatile compounds within their rings in the cosmetics and chemical supply industries as well as to stabilize and protect flavor molecules in the food industry. (Del Valle, 2004).
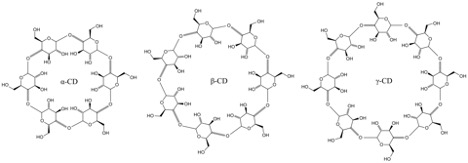
Figure 2: The structures of the three most common cyclodextrins (also called CDs), α-CD, β-CD, and γ-CD
Source: Wikimedia Commons
Due to their stability, lack of toxicity, and the fact that many viruses use sugars on cell membranes to bind and infect host cells, these sugar polymers are attractive antiviral candidates. Previously, researchers demonstrated that sulfonated cyclodextrins (i.e., where the -OH of glucose is replaced with long carbon chains terminating in an SO3– group) had the ability to reversibly bind to and temporarily inhibit HIV. However, upon dilution (what would occur in the blood), this effect was lost (Anand et. al., 1990). Nonetheless, the proof of concept of using sugars to bind and inhibit viruses was demonstrated. It just needed to be made more permanent. Combining this finding with their previous work using potentially toxic gold nanoparticles that were coated in sulfonate groups to permanently bind and destroy multiple different kinds of viral virions (individual virus particles), Jones and colleagues at the University of Manchester developed a sulfonated β-CD they call CD-1 (Jones et al., 2020).
The team in Manchester demonstrated that CD-1 was able to bind and rip open the membranes of the viruses responsible for viral pneumonia (RSV-A and RSV-B), the Hepatitis C Virus (HCV), the H3N2 strain of the flu virus, the Herpes viruses (HSV-1, HSV-2, and even a virucidal resistant strain of HSV-2), and many others. Additionally, CD-1 was able to prevent the infection of B-cells (one target of the virus) in HIV. Furthermore, its effect was not lost after dilution with more aqueous solution, proving that its effect (unlike many virucidal compounds) is irreversible. As an added bonus, no toxicity was seen in host cells that were treated with the compound (Jones et al., 2020).
The authors demonstrated that the drug lost its activity when treated in a solution of fetal bovine blood (potentially due to the effect of other host proteins in the blood), but argue that it could potentially be modified for eventual use in blood by further research. Despite the lack of serum activity, they demonstrated that in its current form, it is perfect for use as a topical treatment. Developing the idea of topical application, Jones’s team demonstrated that CD-1 was still effective in preventing viral infection in cultures of human vaginal tissue cells. and when used as an intravaginal gel in mice. This indicates that the sugar derivative could be used as a vaginal microbicide to help prevent the spread of viral STDs such as HIV, Herpes, or HPV (Jones et al., 2020).
As one last potential benefit to the drug, the authors noted that CD-1 seems to be difficult for viruses to adapt to via mutation. Unlike the approved virucidal drug acyclovir, CD-1 showed no drop in effectiveness after repeated treatment on the same culture of virus cells. This is potentially due to its rapid, permanent manner of action. There is simply no time for the virus to mutate around the drug before it is ripped open. Thus, it would be an attractive drug for use against drug-resistant viruses or as a part of a “drug cocktail” against viruses known to quickly develop resistance to antivirals (such as HIV) (Jones et al., 2020).
Thus, unlike many virucides, CD-1 would have broad-spectrum activity against many viruses, be able to irreversibly bind its targets, be effective on highly mutable and drug-resistant viruses, and likely have little to no adverse effects on patients. While some doctors may be asking you to cut back on your sugar intakes, it’s very possible that someday soon, your doctor may prescribe you some (modified) sugar to fight off that pesky cold or protect yourself from STDs.
References
Anand, R., Nayyar, S., Pitha, J., & Merril, C. R. (1990). Sulphated Sugar Alpha-Cyclodextrin Sulphate, a Uniquely Potent Anti-HIV Agent, Also Exhibits Marked Synergism with AZT, and Lymphoproliferative Activity. Antiviral Chemistry and Chemotherapy, 41–46. https://doi.org/10.1177/095632029000100107
Del Valle, E. M. M. (2004). Cyclodextrins and their uses: A review. Process Biochemistry, 39(9), 1033–1046. https://doi.org/10.1016/S0032-9592(03)00258-9
Jones, S. T., Cagno, V., Janeček, M., Ortiz, D., Gasilova, N., Piret, J., Gasbarri, M., Constant, D. A., Han, Y., Vuković, L., Král, P., Kaiser, L., Huang, S., Constant, S., Kirkegaard, K., Boivin, G., Stellacci, F., & Tapparel, C. (2020). Modified cyclodextrins as broad-spectrum antivirals. Science Advances, 6(5), eaax9318. https://doi.org/10.1126/sciadv.aax9318
University of Manchester. (2020). Unique new antiviral treatment made using sugar. ScienceDaily. Retrieved February 13, 2021 from www.sciencedaily.com/releases/2020/01/200129143339.html
Related Posts
Mixed fungal cultures: a more potent method of crude oil biodegradation
A diagram showing how a fungal cell combines with the...
Read MoreHow the Human Microbiome Disables Drugs (And What We Can About It)
Scientists are studying the human microbiome and its ability to...
Read MoreFrankie Carr