
The pliability and low-cost of synthetic thermoplastics have rendered them nearly irreplaceable in the modern world. The discovery of polymeric materials was an incredible feat for humankind. But in spite of their many favorable properties, one thing has become abundantly and alarmingly clear: they are incapable of degradation.
The rate at which we discard these materials, coupled with the colossal amount annually produced anew, make an unsettling pair. Only 30% of PET plastic is successfully recycled each year in the US, despite being arguably the most recyclable plastic in public circulation [1]. Upon collection, the “garbage” merely requires a system of sorting, washing, grinding and melting processes to retain its initial, seemingly-untouched form.
PET is primarily used in single-use beverage containers due to its inert properties and material strength, but can also be found in fabrics, construction materials, or various packaging applications [1]. It’s a very versatile polymer with many favorable qualities, yet its environmental damage can no longer be overlooked as pollution rates rise and feedstocks of raw, non-renewable materials diminish. Jim Dumesic, a Chemical and Biological Engineering professor at UW-Madison, has developed a system in which biomass, rather than crude oil and natural gas, feeds the synthesis reaction of a material practically indistinguishable from PET, called polyethylene furanoate (PEF).
PEF is created by the synthesis of ethylene glycol and furandicarboxylic acid (FDCA). Forming high concentrations of this latter component normally requires the addition of a homogenous base to improve its solubility, resulting in the formation of unwanted salts in the effluent of the reactor that require further separation and resources. However, the Dumesic Research Group discovered that the presence of a plant-derived solvent denoted GVL (γ-valerolactone) enabled a high solubility of FDCA, alleviating the need for a homogenous base [2]. Conducting the dehydration reaction of fructose, a natural sugar, in this organic mixture along with some of the desired product itself (FDCA) resulted in a high yield of FDCA’s precursor, HMF. They applied a Pt/C catalyst to the system in place of a corrosive mineral acid, as often used in fructose dehydration reactions, after finding they could recover and recycle it. HMF, when subsequently oxidized in these conditions, formed a high concentration of FDCA without the formation of waste products as aforementioned. This system introduces PEF as a viable replacement to PET as it is similar in cost and richer in properties, without the environmental baggage.
This bio-renewable plastic has a lower melting temperature and a higher glass transition temperature than that of PET. This means that PEF can be morphed at less extreme conditions in its unit operations and has the ability to withstand higher temperatures without losing its structural integrity. In other words, manufacturing sites would reduce their energy consumption and product applications could expand, yielding more profit. Further, regulatory experiments have proven that PET is much more susceptible to oxygen and carbon dioxide permeation than PEF, making PEF a much more attractive candidate in applications concerning food packaging.
Though both require enzymatic treatment to induce degradation, replacing PET with PEF would greatly reduce associated greenhouse gas emissions as well as our collective dependence on nonrenewable resources. UC-Berkeley is currently conducting research on the manipulation of PETase (a PET-digesting enzyme) to lower its cost and alter its substrate specificity to encompass and degrade a variety of synthetic plastics, which would revolutionize the modern world [3]. This development would reduce current plastic waste and its effects while entirely halting its accumulation. Further, the Huber Research Group under CBE Professor George Huber at UW-Madison is working to optimize plant biomass conversion pathways with the goal to develop cleaner fuels and chemicals via more economical processes [4]. Shifting from a petroleum-dependent world is no longer a distant dream.
The world is at an immensely pivotal moment in time. Anthropogenic environmental degradation has rendered many ecosystems uninhabitable over the years, soon to be ours. Unfortunately, polymeric materials add to the destruction. Who says they have to? Deriving the bulk of our packaging pollution from biomass is a sizeable way to reduce environmental harm. Constructing enzymes to reduce current and future waste accumulation will be another. Truly significant sustainable development is on the rise and climbing, yet much is still to be done.
References
- How Plastics Are Made. Available: https://plastics.americanchemistry.com/How-Plastics-Are-Made/
- Made HI. YouTube. YouTube; 2013. Available: https://www.youtube.com/watch?v=ed7XJeXl3b4
- Motagamwala AH, Won W, Sener C, Alonso DM, Maravelias CT, Dumesic JA. 2018. Science Advances, 4(1): Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5775026/
- Welcome to the Dumesic Research Group. Index. Available: http://jamesadumesic.che.wisc.edu/
- Austin HP, Allen MD, Donohoe BS, Rorrer NA, Kearns FL, Silveira RL, et al. 2018 Characterization and engineering of a plastic-degrading aromatic polyesterase. PNAS, 115 (19) E4350-E4357. Available: http://www.pnas.org/content/early/2018/04/16/1718804115
- Huber Group Research. Huber Group Research. Available: http://biofuels.che.wisc.edu/
Related Posts
The Brain and Sign Language — Evidence of a Universal Language Center?
Figure 1: The English alphabet and numbers 1-9 as signed...
Read MoreThe “Old Friends” Hypothesis and the COVID-19 Pandemic
Authors: Sakeena Badrane1, Vivek Babu2, Sanah Handu1 1University of Pittsburgh,...
Read MoreThe Next Source of Antibiotics: Viruses
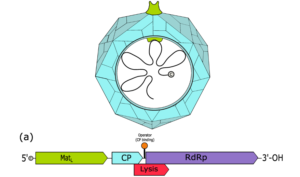
Figure 1: A computer-generated image of a bacteriophage called a...
Read MoreAislen Kelly

Comments are closed.