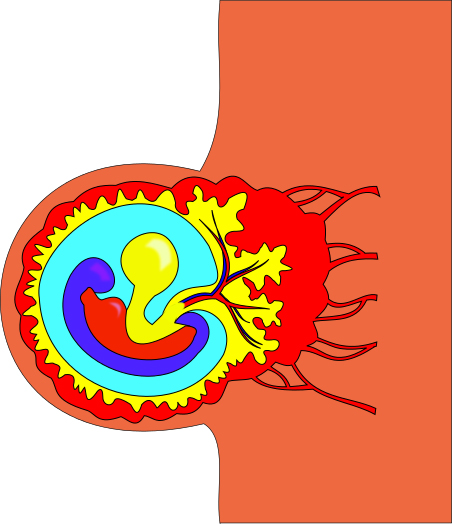
Figure 1: Researchers at the Planck Institute for Molecular Genetics in Berlin have formulated a new method of studying epigenetic factors during the gastrulation period in mice. The gastrulation period, shown in the above image, is an early stage of development in which the body axes and germ layers form. Before gastrulation, which typically occurs around week three in human development, only a blastula exists.3
How does mere DNA instruct the development of a whole organism from its start as a single cell? Many biology students have wondered this after an introduction to the human species’ 23 chromosomes. A key part of this answer surrounds the study of epigenetics, which involves DNA modifications that do not affect the genetic sequence, but alter the expression frequency of the genes in DNA.4 Epigenetics plays a fundamental role in developmental biology, determining how cells in early development will differentiate – into lung, skin, or heart cells – despite having the same DNA. Notably, embryos without the necessary epigenetic factors will not grow past the embryonic period.1 Therefore, targeted inquiry into what these epigenetic factors are, how they work, and how they lead to differentiated cells is crucial.
Researchers at the Planck Institute for Molecular Genetics in Berlin have recently formulated a new method of studying epigenetics, allowing for the exploration of mutant embryos both anatomically and molecularly.1 The study involved transcriptomic analysis (sequencing mRNA, the message decoded from DNA and translated into protein) of 88,779 cells from 50 wild-type embryos compared to 8 – 12 mutant embryos of 7,548 – 25,408 each. Mutant embryos were created by disrupting one epigenetic regulator protein per organism during early and mid-gestation. The epigenetic regulator proteins of choice were the three major DNA methyltransferases (DNMT1, DNMT3A, DNMT3B) and the repressive histone 3 lysine 9 (H3K9) methyltransferase G9A. These regulators were selected because of their known significant role in development—mutations in the targeted regulators result in death either after gastrulation or postnatally.2
The data allowed the researchers to assign mutant cells by marker expression to their corresponding wild-type state and examine embryo development alongside transcriptional state.2 Both genotype and phenotype were examined, a significant advancement in epigenetic research as many others focus on target phenotype.1 In addition, the research created the opportunity for further examination of what each regulator is responsible for within development, whether it be functioning as neural regulators, germline creation regulators, or tumor suppressors. The study confirmed historical observations and brought new findings to the table about when and how mutations in these key epigenetic regulators start affecting development.2 As noted by lead investigator Alexander Meissner, such questions about development have been circling in biological research for 25 years and this study was only the beginning for further study. Using the methods in this study, observations of early developmental stages can now be made on “previously unthinkable” levels of detail.1
References
- Cell diversity in the embryo: Epigenetic factors control the development of an organism. ScienceDaily. (2020). Retrieved 14 August 2020, from https://www.sciencedaily.com/releases/2020/08/200804111532.htm.
- Grosswendt, S., Kretzmer, H., Smith, Z., Kumar, A., Hetzel, S., & Wittler, L. et al. (2020). Epigenetic regulator function through mouse gastrulation. Nature. Retrieved 14 August 2020, from https://www.nature.com/articles/s41586-020-2552-x.
- Hill, M. (2020). Gastrulation – Embryology. Embryology.med.unsw.edu.au. Retrieved 14 August 2020, from https://embryology.med.unsw.edu.au/embryology/index.php/Gastrulation.
- What is epigenetics?. Genetics Home Reference. (2020). Retrieved 14 August 2020, from https://ghr.nlm.nih.gov/primer/howgeneswork/epigenome.
Related Posts
An Introduction to the Ebola Virus
Cover Image: Blood sample of a patient infected by the...
Read MoreContradicting the Disparity Between Primate and Avian Brains
Figure 1: A Northwestern Crow near Whittier, Alaska. Corvids, along...
Read MoreConcierge Medicine Could Reduce Senior Health Inequities in Illinois
This publication is in proud partnership with Project UNITY’s Catalyst Academy 2023...
Read MoreLove Tsai



