Figure 1: The representation of cellular activity of SARS-COV-2 as it passes throughout the human body and looks to contaminate organs.
Source: Wikimedia Commons
The novel coronavirus, otherwise known as SARS-CoV-2, belongs to the broader classes of coronaviruses (some of which, like SARS-CoV-1 and MERS, have a known history of causing widespread death). After performing cellular adhesion and cellular replication, the coronavirus looks to mobilize and travel throughout organ systems of the human body. Subsequently, the human cells invaded by the coronavirus look to immediately inform the immune system about a pathogen that has entered into the body. The coronavirus tactically aims to inhibit this from happening and sustain infection. The focus of this paper is to analyze the immediate immunological and physiological effects of the novel coronavirus.
Immediate immunological effects of coronavirus replication
The coronavirus is able to replicate and produce new coronavirus cells within a human’s organ systems. Being a respiratory disease, the coronavirus has high affinity for the lungs, and affects both the bronchi and pleura (Patel, 2020). The coronavirus causes extreme alveolar damage once it is in the lungs, causing further damage towards epithelial cells and tissue and therein diminishing lung function, which tends to cause dry cough, fever and sometimes pneumonia (Sims et al., 2005). Nevertheless, the most serious effect of virus in the lungs is the diagnosis of acute respiratory distress syndrome (ARDS) (Radcliffe, 2020). ARDS is a respiratory illness that deprives organs of the necessary levels of oxygen required to function (ARDS – Symptoms and Causes, 2020). Specifically, ARDS regards the case of fluid leakage from the small blood vessels inside the lungs. This fluid builds up in the alveoli of the lungs and prevents them from containing the proper distribution of oxygen, making oxygenation of tissues more difficult and in some instances leading to death (Radcliffe, 2020).
Although the immune system responds to coronavirus infection of the lungs, a coronavirus virion can prevent the host cell from sending ligands that inform the immune system of the trespassing virus (Patel, 2020). The immune system is then rendered unable to deliver its assistance and guidance to eliminating the coronavirus because it is not notified by the host cell that was initially infected. However, this is not always case. The degree of awareness of the immune system is dependent on many coronavirus cells present within an organ system at the time of ligand reception by the immune system (Hogan, 2020). For instance, if the there are only a few coronavirus virions present, the immune system will receive few ligands from the host cell (but usually enough to recognize that the body is under attack) (Hogan, 2020). If there are many coronavirus cells, the immune system will recognize coronavirus as a dangerous pathogen (Hogan, 2020).
Biologically, the immune system is capable of eliminating the coronavirus by secreting immune messengers known as cytokines (Radcliffe, 2020). Cytokines are very small proteins secreted by the immune system that instigate proinflammatory effects and help (Tisoncik et al., 2012). However, the immune system is also prone to making a certain critical mistake when attempting to defend the human from the coronavirus (Radcliffe, 2020). The constant first response to coronavirus presence by the immune system is to secrete cytokines,but the immune system sometimes secretes an excessive amount of cytokines – a so-called “cytokine storm” (Tisoncik et., 2012), Ultimately, the occurrence of a cytokine storm can cause extreme inflammation to tissues and other organs such as the heart, blood vessels, liver, and many more (“COVID-19 basics”, 2020).
A newly replicated coronavirus will also productively evade innate immune response (Patel, 2020). Innate immunity is a series of nonspecific defense mechanisms that allow for destruction of pathogens (“Introduction to Immunology Tutorial”, 2000). These defense mechanisms include toll-like receptors, interferons, and the complement system (Birra et al., 2020).
Toll-like receptors (TLs) are a combination of eleven transmembrane receptor proteins that quickly acknowledge any pathogen-associated molecular pathways, allowing them to locate the coronavirus’s initial movement (Birra et al., 2020). Out of the eleven, one toll-like receptor is responsible for locating and developing a protective response specifically against the coronavirus (Birra et al., 2020). This specific toll-like receptor, TL3, will act by first identifying a certain host cell hijacked by the coronavirus (Vercammen et al., 2008). TL3 will then diffuse through the phospholipid bilayer of the host cell, and immediately destroy the initial uncoated viral RNA in the cytoplasm to halt viral biosynthesis (Vercammen et al., 2008).
Interferons (IFN) are a group of three signaling proteins, type I (IFNαβ), type II (IFNϒ), and type III (IFNλ) that all play critical roles in a human’s innate immunity as they deliver various immune responses related to combating against the coronavirus (Birra et al., 2020). Out of the three interferons, type I (IFNαβ) is most active in coronavirus resistance and sends two cytokines to the initial host cell that has been infected (Birra et al., 2020). After arrival, the two cytokines diffuse through the phospholipid bilayer of the host cell and immediately destroy the uncoated viral RNA in the cytoplasm (Birra et al., 2020). This will put an end to all biosynthetic processes the coronavirus is taking to advance the replication process.
However, the coronavirus will send its M and N proteins to fully block the interferon cell signaling pathway which will inhibit them from reaching the virus-infected cells in the respiratory tract (Siu et al., 2009, Gonzalez-Navajas et al., 2012). SARS-COV-2 M and N proteins block around 92-97% of all types of interferons from entering the cell signaling pathway to perform their defense mechanisms (Siu et al., 2009, Birra et al., 2020). This allows almost no interferons to enter, making the coronavirus capable of continuing replication on host cells and ultimately invading the lungs and immune system of the human.
A complement system is a group of plasma proteins that react with each other to produce a series of inflammatory responses that eliminate infectious pathogens (Janeway, 2020). It accomplishes this via opsonization, the process of identifying and marking a foreign pathogen to be eliminated by phagocytes (Thau, 2020). Once infected cells are marked, a large group of phagocytes will attach and metabolize the coronavirus cells (Java et al., 2020). Unfortunately, the coronavirus can evade the complement system’s antiviral process as well. It does so by producing two microproteins, known as C3a and C5a, that will attach to the coronavirus particle and prevent phagocyte attachment (Li, 2020).
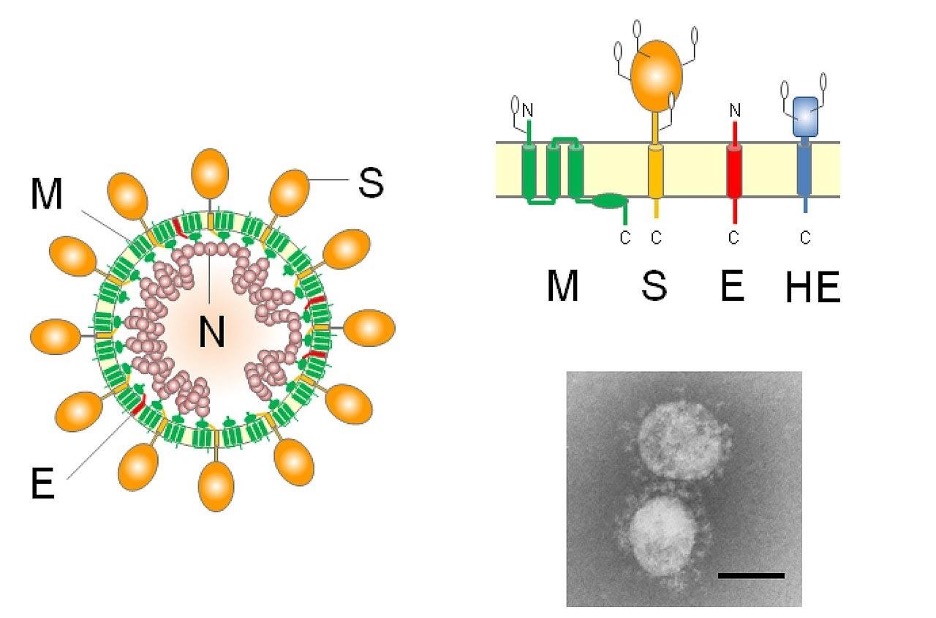
Figure 2: Overall anatomical structure of the coronavirus cell. Specifically, the M and N proteins inhibit biomolecular synthesis and presence of the interferons in the cell signaling pathway.
Image Source: Wikimedia Commons
Immediate physiological effects of coronavirus replication
The coronavirus, a firm threat for the lungs, is also undoubtedly capable of affecting more organs throughout a human, with severe effects. When the coronavirus looks to replicate, it utilizes a specific membrane receptor enzyme known as angiotensin converting enzyme 2 (ACE-2) to enter the human cell. Additionally, another enzyme known as transmembrane protease receptor enzyme 2 (TMPRSS2), regulates the attachment of the coronavirus to ACE-2 by properly allowing the coronavirus to travel across the phospholipid bilayer and into the cytosol of the cell for replication. As the coronavirus performs constant replication, it will then eventually begin to contact pathways to peripheral nerves and blood as those are the standard routes that will carry the coronavirus from one organ to another (Baron et al., 1996). These further organs that the coronavirus is capable of traveling to include the heart, blood vessels, liver, kidneys, stomach, intestines, brain, bone marrow, spleen, and the male prostate gland. Looking at patients, 27-30% experience inflammation to the heart and blood vessels, 10-15% experience inflammation to the liver, stomach, intestines, or kidneys, and around 5% experience inflammation toward the brain, spleen, or bone marrow. (“Coronavirus and Heart Disease – Penn Medicine”, 2020). The remaining majority of the infected population sees most inflammation in the lungs and the respiratory system. As with any virus, this inflammation can be minor or severe, which is primarily dependent on the physiological structure of the infected human.
Heart: Aside from lung and immune system damage, cardiovascular damage is the most common effect of coronavirus infection. Individuals with heart disease are particularly susceptible, but people without a history of prior diagnosed cardiovascular disease are still able to have their hearts contaminated with coronavirus cells. The invasion of the heart may cause severe inflammation of heart muscles and arteries surrounding the heart that are responsible for carrying blood (Pesheva, 2020). This inflammation can directly cause cardiac muscle damage, weakening the ability of the heart to pump a proper amount of blood, and lead to heart rhythm disruptions (Pesheva, 2020).
There are a few specific detrimental effects of coronavirus infection on heart function. First, as the coronavirus instigates inflammation of the heart, fluid may fill up the alveoli of the lungs, causing minimal oxygen to reach the bloodstream (Michos, 2020). The heart now has to exert extreme amounts of effort to pump blood throughout the entire body; moreover, this exertion of effort can cause the heart to overwork itself to maintain proper amounts of oxygen, causing an immediate heart attack, or the lack of oxygen present may simply cause cell and tissue damage in the heart (Michos, 2020). Second, the coronavirus infects the heart muscle tissue and the cells within them, causing a life-threatening inflammatory reaction known as myocarditis (Michos, 2020). Myocarditis will damage the heart tissues and cells, prevent it from accurately pumping blood, and ultimately cause heart failure (“Coronavirus and Heart Disease – Penn Medicine”, 2020). Third, the coronavirus can attack heart muscles and cause the heart to be unable to pump blood, producing a heart muscle disorder known as stress cardiomyopathy (Michos, 2020). Stress cardiomyopathy begins once the human is infected by the coronavirus because a human will go through immediate stress, and release hormones from the adrenal gland known as catecholamines to ease that stress (Michos, 2020). However, catecholamines will shock the heart, causing debilitated blood pumping and misuse of oxygen. Fourth, the coronavirus can cause an increase in blood clots throughout the body (“Coronavirus and Heart Disease – Penn Medicine”, 2020). These blood clots produce heavy bleeding and clotting throughout small blood vessels and pathways in the heart (“Coronavirus and Heart Disease – Penn Medicine”, 2020). An oversupply of blood clots can lead to stroke and heart attack.
Figure 3: Infection of the heart by coronavirus can lead to several detrimental effects – including myocarditis and stress cardiomyopathy.
Image Source: Wikimedia Commons
Blood Vessels:
Once in the blood vessels, the coronavirus cells will bind to the ACE-2 receptors within the vascular tissues and start infecting the entirety of endothelial cells that form the lining of the blood vessels (Metacic, 2020, Beeland et al., 2020). This instigates a local immune response and ultimately inflames the endothelium (Smith, 2020). Endothelial cells have the important function of inhibiting blood clot formation by releasing proteins that ensure that there is smooth blood flow and no plaque buildup aside the walls of the blood vessels (Smith, 2020). Therefore, the elimination of endothelial cells by the coronavirus will cause a series of detrimental effects on the flow of blood throughout all blood vessels within a human.
This damage includes inflammation in the blood vessels and blood vessel damage which would cause the plaque aside the walls of the blood vessels to rupture and cause blood flow to the heart to heavily weaken, making the risk of a heart attack very high (Smith, 2020). Also, the infection of endothelial cells will cause blood vessels to leak and produce a serious supply of blood clots (Matacic, 2020). These blood clots will provide a stoppage of accurate blood flow in certain portions of the blood vessels of a human due to the heavy plaque buildup. Those changes will ultimately cause inflammation and, as previously described, fuel the severity of ARDS (Matacic, 2020). Additionally, blood vessels will begin to abnormally grow with the presence of the coronavirus (St. Peter, 2020).
The vascular system does possess a healing response to the damage caused by the coronavirus (Hudson, 2020). This healing response is known as intussusceptive angiogenesis which is a mechanism responsible for repairing inefficient blood vessels and producing new blood vessels (Mentzer et al., 2014, Hudson, 2020). It conducts this process by distributing interstitial tissues into existing blood vessels that form transvascular tissue pillars that expand and produce a blood vessel capable of accurately moving blood throughout a human (Adair, 2020).
Figure 4: The process of intussusceptive angiogenesis, in which blood vessels are being prepared and produced. Unfortunately, the coronavirus produces severe blood clots in these blood vessels preventing this repairing process of angiogenesis from occurring.
Image Source: Wikimedia Commons
Liver: The susceptibility of a human to liver damage due to the coronavirus is small, but it can very well be high if the human has prior diagnosed diseases that have weakened the liver and its bile production. Cirrhosis, for example, is a long-term disease that builds up scar tissue that replaces healthy liver tissue, causing a weakened flow of blood through the liver (“Chronic Liver Disease/Cirrhosis”, 2020). However, any human, no matter their medical profile, is prone to suffering from liver damage if infected with the coronavirus. First, ACE-2 levels within the liver are typically very high, which means the liver attracts coronavirus cells at a higher rate than other organs and it is a location where the coronavirus can grow and replicate (Pass, 2020) The coronavirus will utilize the mitochondria of liver cells to strategically distort their functions of energy synthesis, immune signaling, and cellular metabolism to facilitate its proliferation (Khan et al., 2015). This interferes with the cellular signaling pathways in the liver and heavily cripples innate immune signaling, rendering the immune system incapable of handling the coronavirus present within the liver (Khan et al., 2020).
First, the level of liver enzymes rapidly increases, which demonstrates that the liver is being damaged (Coronavirus Disease COVID-19, 2020). Once oversupplied, the liver will secrete a specific enzyme, known as aspartate aminotransferase (AST) into the bloodstream, as this enzyme is an indicator of other organs that the liver cells have been attacked by a pathogen and are suffering from inflammation (Coronavirus Disease COVID-19, 2020, AST Test, 2020). Second, congestive hepatopathy can result, which is the state of congestion of the liver (Hilscher et al., 2016, Schaefer, 2020). Third, cirrhotic cardiomyopathy is also a result of coronavirus activity as this is a disease that damages systolic response of the body to physiological abnormalities (as seen instigated by the coronavirus), physical stress, and diastolic dysfunction (Chayanupatkul et al., 2014, Schaefer et al., 2020).
Stomach and Intestines: Patients with inflammatory bowel disease (IBD) suffer from inflammation in their large intestine. This condition promotes coronavirus susceptibility by increasing ACE-2 expression within the walls of the large intestine (Inflammatory Bowel Disease (IBD) – symptoms and causes, 2020). All humans are susceptible to suffering from intestinal damage regardless of prior diagnosed disease. ACE-2 and TMPRSS2 count is extremely high in the absorptive enterocytes of the ileum, the middle part of the small intestine, and the entirety of the large intestine, which allows the coronavirus to settle into the intestines and begin infecting the healthy cells (Samanta et al., 2020, Ma et al., 2020). Moreover, the intestines are capable of activating their interferon-stimulated genes to defend themselves from a viral infection; but, the number of infected intestine cells quickly increases primarily due to the abundance of ACE-2 and TMPRSS2, which does not allow the intestines to accurately implement their defense strategy against the coronavirus (Coronavirus SARS-CoV-2 infects cells of the intestine). The coronavirus activity toward the intestines has harmful effects that may lead to other severe diseases. Inflammation from the coronavirus to the intestines is so severe that the intestinal epithelium begins to suffer from direct damage (Samanta et al., 2020). This direct damage is so severe that the human will most likely develop alternating segmental dilation and stenosis of the small intestine (Ma et al., 2020). In addition, the intestinal flora may very well become imbalanced which could lead to miscontrol of digestion (Samanta et al., 2020).
Conclusion
The immunological and physiological effects due to the coronavirus, both short-term and long-term are significant in many individuals as these effects are present within the immune system, lungs, and many more organs. SARS-CoV-2 is a novel virus, prompting the fact that the immune system and the human defense mechanisms will perform all capable strategies to eliminate the coronavirus. Moreover, the biological strength, unwillingness to resist its objective, and strategic organization within the human body on the part of the coronavirus, demonstrates the classification and venture of the coronavirus towards the immunological and physiological components of the human body.
References
Adair, T. H. (2020, August 22). Overview of Angiogenesis – Angiogenesis – NCBI Bookshelf. National Institute of Health. https://www.ncbi.nlm.nih.gov/books/NBK53238/
AST Test. (2020). MedicinePlus. https://medlineplus.gov/lab-tests/ast-test/
Baron, S., Fons, M., & Albrecht, T. (1996, August 22). Viral Pathogenesis – Medical Microbiology – NCBI Bookshelf. National Institute of Health. https://www.ncbi.nlm.nih.gov/books/NBK8149/
Beeland, D., & Emerging Pathogens Institute. (2020, June 5). Autopsies of COVID-19 victims reveal blood vessel damage. University of Florida. https://www.epi.ufl.edu/articles/covid-19-blood-vessel-damage.html
Bilinska, K., Jakubowska, P., Bartheld, C. S. V., & Butowt, R. (2020, May 7). Expression of the SARS-CoV-2 Entry Proteins, ACE2 and TMPRSS2, in Cells of the Olfactory Epithelium: Identification of Cell Types and Trends with Age. American Chemical Society. https://pubs.acs.org/doi/10.1021/acschemneuro.0c00210
Birra, D., Benucci, M., Landolfi, L., Merchionda, A., Loi, G., Amato, P., Licata, G., Quartuccio, L., Triggiani, M., & Moscato, P. (2020, May 10). COVID 19: a clue from innate immunity. PubMed Central (PMC). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7286633/
Center for Disease Control. (2020, February 11). Coronavirus Disease 2019 (COVID-19). Centers for Disease Control and Prevention.
Chayanupatkul, M., & Liangpunsakul, S. (2014, September 11). Cirrhotic cardiomyopathy: review of pathophysiology and treatment. PubMed Central (PMC). https://pubmed.ncbi.nlm.nih.gov/25221635/
Coronavirus Disease 2019 (COVID-19). (2020, February 11). Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/liver-disease.html
Gonzalez-Navajas, J. M., Lee, J., David, M., & Raz, E. (2012, January 6). Immunomodulatory functions of type I interferons. PubMed Central (PMC). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3727154/
Harvard Health Publishing. (2020, August 21). COVID-19 basics. Harvard Health. https://www.health.harvard.edu/diseases-and-conditions/covid-19-basics
Hilscher, M., & Sanchez, W. (2016, October 2). Congestive hepatopathy. PubMed Central (PMC). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6490201/
Hogan, A. (2020, April 13). How much of the coronavirus does it take to make you sick? The science, explained. STAT. https://www.statnews.com/2020/04/14/how-much-of-the-coronavirus-does-it-take-to-make-you-sick/
Hubrecht Institute. (2020, May 4). Coronavirus SARS-CoV-2 infects cells of the intestine. ScienceDaily. https://www.sciencedaily.com/releases/2020/05/200504091438.htm
Hudson, J. (2020, May 28). New study shows COVID-19 causes severe blood vessel damage. Venous News. https://venousnews.com/new-study-shows-covid-19-causes-blood-vessel-damage/
Inflammatory bowel disease (IBD) – Symptoms and causes. (2020, March 3). Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/inflammatory-bowel-disease/symptoms-causes/syc-20353315
Janeway, C. A. (2020, August 21). The complement system and innate immunity – Immunobiology – NCBI Bookshelf. National Institute of Health (NIH). https://www.ncbi.nlm.nih.gov/books/NBK27100/
Java, A., Kim, A. H. J., Kulkarni, H. S., Apicelli, A., Liszewski, M. K., Coler-Reilly, A., & Atkinson, J. P. (2020). JCI Insight – The complement system in COVID-19: friend and foe? JCI Insight. https://insight.jci.org/articles/view/140711/pdf
Johns Hopkins Medicine. (2020). Chronic Liver Disease/Cirrhosis. https://www.hopkinsmedicine.org/health/conditions-and-diseases/chronic-liver-disease-cirrhosis
Khan, M., Syed, G. H., Kim, S. J., & Siddiqui, A. (2015, October 1). Mitochondrial dynamics and viral infections: A close nexus. ScienceDirect. https://www.sciencedirect.com/science/article/pii/S0167488915000099
Li, G., Fan, Y., Han, T., Li, Z., Zhou, P., Pan, P., Wang, W., Hu, D., Liu, X., Zhang, Q., & Wu, J. (2020, January 25). Coronavirus infections and immune responses. Journal of Medical Virology. https://onlinelibrary.wiley.com/doi/10.1002/jmv.25685
Ma, C., Cong, Y., & Zhang, H. (2020, July). COVID-19 and the Digestive System : Official journal of the American College of Gastroenterology | ACG. LWW. https://journals.lww.com/ajg/Fulltext/2020/07000/COVID_19_and_the_Digestive_System.11.aspx
Matacic, C. (2020, June 2). Blood vessel attack could trigger coronavirus’ fatal ‘second phase.’ Science | AAAS. https://www.sciencemag.org/news/2020/06/blood-vessel-attack-could-trigger-coronavirus-fatal-second-phase
Mayo Clinic. (2020, June 13). ARDS – Symptoms and causes. https://www.mayoclinic.org/diseases-conditions/ards/symptoms-causes/syc-20355576
Mentzer, S., & Konerding, M. (2014, March 26). Intussusceptive Angiogenesis: Expansion and Remodeling of Microvascular Networks. PubMed Central (PMC). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4063884/
Michos, E. D. (2020, April 24). Can Coronavirus Cause Heart Damage? Johns Hopkins Medicine. https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/can-coronavirus-cause-heart-damage
National Heart, Lung, and Blood Institute. (2019, January 23). Peripheral Artery Disease | NHLBI, NIH. National Institute of Health. https://www.nhlbi.nih.gov/health-topics/peripheral-artery-disease
Pass, W. (2020, March 19). Patients with COVID-19 may face risk for liver injury. CHEST Physician. https://www.mdedge.com/chestphysician/article/219309/coronavirus-updates/patients-covid-19-may-face-risk-liver-injury
Patel, N. (2020, April 15). How does the coronavirus work? MIT Technology Review. https://www.technologyreview.com/2020/04/15/999476/explainer-how-does-the-coronavirus-work/
Penn Medicine. (2020, June 3). Coronavirus and Heart Disease – Penn Medicine. https://www.pennmedicine.org/updates/blogs/heart-and-vascular-blog/2020/june/coronavirus-and-heart-disease
Pesheva, E. (2020, April 13). Coronavirus and the Heart. Harvard Medical School. https://hms.harvard.edu/news/coronavirus-heart https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html
Radcliffe, S. (2020, April 30). Here’s What Happens to the Body After Contracting the New Coronavirus. Healthline. https://www.healthline.com/health-news/heres-what-happens-to-the-body-after-contracting-the-coronavirus#COVID-19-affects-lungs
Samanta, J., Dhar, J., Khaliq, A., & Kochhar, R. (2020, May 16). 2019 Novel Coronavirus Infection: Gastrointestinal Manifestations. PubMed Central (PMC). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7295271/
Schaefer, E. A. K., Arvind, A., Bloom, P., & Chung21, R. T. (2020, May 21). Interrelationship Between Coronavirus Infection and Liver Disease. Clinical Liver Disease, a Multimedia Review Journal. https://aasldpubs.onlinelibrary.wiley.com/doi/10.1002/cld.967
Sims, A., Baric, R., Yount, B., Burkett, S., Collins, P., & Pickles, R. (2005, December 1). Severe Acute Respiratory Syndrome Coronavirus Infection of Human Ciliated Airway Epithelia: Role of Ciliated Cells in Viral Spread in the Conducting Airways of the Lungs. PubMed Central (PMC). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1316022/
Siu, K. L., Kok, K. H., Ng, M. H. J., Poon, V. K. M., Yuen, K. Y., Zheng, B. J., & Jin, D. Y. (2009, June 12). Severe Acute Respiratory Syndrome Coronavirus M Protein Inhibits Type I Interferon Production by Impeding the Formation of TRAF3·TANK·TBK1/IKKϵ Complex. PubMed Central (PMC). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2713514/
Smith, D. G. (2020, May 29). Coronavirus May Be a Vascular Disease, Which Explains Everything | Elemental. Medium. https://elemental.medium.com/coronavirus-may-be-a-blood-vessel-disease-which-explains-everything-2c4032481ab2
St. Peter, E. (2020, May 28). Distinctive Features. Harvard Medical School. https://hms.harvard.edu/news/distinctive-features
Stevens, R. (2020, June 4). How Does Coronavirus Affect the Brain? Johns Hopkins Medicine. https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/how-does-coronavirus-affect-the-brain
Tisoncik, J., Korth, M., Simmons, C., Farrar, J., Martin, T., & Katze, M. (2012, March 1). Into the Eye of the Cytokine Storm. PubMed Central (PMC). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3294426/
Thau, L. (2020, May 24). Physiology, Opsonization – StatPearls – NCBI Bookshelf. National Institute of Health (NIH). https://www.ncbi.nlm.nih.gov/books/NBK534215/
Thrombocytopenia (low platelet count) – Symptoms and causes. (2020, April 8). Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/thrombocytopenia/symptoms-causes/syc-20378293
University of Arizona. (2000, May 24). Introduction to Immunology Tutorial. The Biology Project. http://www.biology.arizona.edu/immunology/tutorials/immunology/page3.html
University of Maryland Medical System. (2020). Coronavirus and Heart Disease. https://www.umms.org:443/coronavirus/what-to-know/managing-medical-conditions/conditions/heart
Vercammen, E., Staal, J., & Beyaert, R. (2008, January 21). Sensing of Viral Infection and Activation of Innate Immunity by Toll-Like Receptor 3. PubMed Central (PMC). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2223843/
What are the Long-Term Effects of COVID-19? (2020, July 21). Health News | University of Miami Hospitals and Clinics. https://news.umiamihealth.org/en/what-are-the-long-term-effects-of-covid-19/
Yang, M., Hon, K. L. E., Fok, T. F., & Li, C. K. (2003, June). The effect of SARS coronavirus on blood system: its clinical findings and the pathophysiologic hypothesis. PubMed. https://pubmed.ncbi.nlm.nih.gov/12844398/
Zhan, J., Deng, R., Tang, J., Zhang, B., Tang, Y., Wang, J. K., Li, F., Anderson, V. M., McNutt, M. A., & Gu, J. (2006, November). The spleen is a target in severe acute respiratory syndrome. PubMed. https://pubmed.ncbi.nlm.nih.gov/17077309/
Sperati, C.J. (2020, May 14). Coronavirus: Kidney damage caused by COVID-19. Johns Hopkins Medicine.https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/coronavirus-kidney-damage-caused-by-covid19
Gu, J., & Korteweg, C. (2007, April 1). Pathology and Pathogensis of Severe Acute Respiratory Syndrome. PubMed Central (PMC). https://pubmed.ncbi.nlm.nih.gov/17077309/
Related Posts
“Living” Droplets Can Produce Renewable Hydrogen
Figure 1: A microscopic image of Chlorella vulgaris, a species...
Read MoreEmotion-Related Impulsivity and Disordered Eating Behaviors: A Literature Review
Figure: An artistic depiction of disordered eating behavior under the...
Read MoreAntimicrobial Resistance in Underprivileged Communities
This publication is in proud partnership with Project UNITY’s Catalyst...
Read MoreArya Dean Faghri