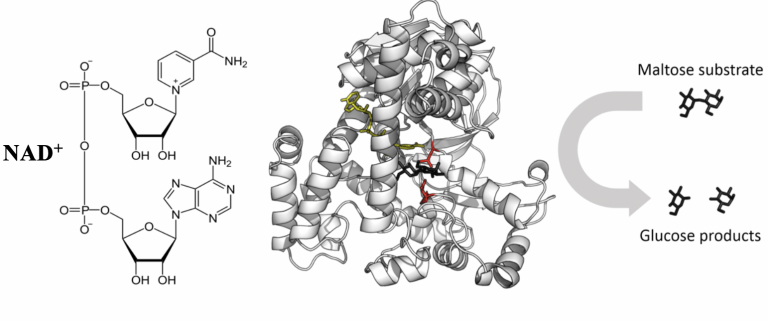
Figure 1: This figure illustrates the interaction between an enzyme, a substrate, and a cofactor. The enzyme is glucosidase, which catalyzes the breakdown of its substrate maltose to glucose. This reaction is facilitated by the cofactor nicotinamide adenine dinucleotide (NAD+). This cofactor is derived from vitamin B3.3 The images above (of NAD+ and glucosidase) have been modified to better suit the purposes of this article.
Ever wonder what allows physiological reactions to occur at rates unimaginable in a lab without the use of extreme conditions and toxic metals? The answer is enzymes. Even the simple act of breathing would be impossible without these molecules.1 It may come as a surprise, but all reactions within our body, even the simplest ones, must be accelerated in order to support life.
The activity of enzymes is analogous to solving a jigsaw puzzle. Say you’re making the face of Rapunzel; if Enzyme A is one half of Rapunzel’s face, then Substrate A is the other complementary half that makes the face whole. Substrates are reagents that are either broken down or combined with other substrates due to the action of enzymes.1 For the most part, only a single type of substrate (Substrate A) can attach itself to the matching enzyme (Enzyme A).1 Specificity in enzymes isn’t only seen with the substrates but also in the reaction the enzyme ultimately carries out.2 Such specificity exists because of the geometric and chemical restraints of the active site (binding pocket on the enzyme), which ensures an energetically favorable interaction between enzyme and substrate(s).2
While the jigsaw puzzle analogy is helpful to understand enzyme specificity, it may create the misleading perception that enzymes are rigid objects. It is important to remember that enzymes, like other molecules in the body, are highly dynamic. When the active site first makes contact with the substrate, it moves in a manner such that it can better accommodate the substrate and hence enhance essential, favorable interactions.2 But the jigsaw analogy is valid in a different way – just as pieces of a jigsaw puzzle require the hands of a solver, enzymes require “helper molecules” to better accomplish their task. Such helper molecules are known as cofactors. A portion of them, specifically known as coenzymes, are derived from the vitamins we consume in our diet.1This makes vitamins crucial molecules, for without them enzymes wouldn’t be functional. Cofactors which aren’t coenzymes are inorganic ions such as iron and magnesium.
Nicotinic acid, commonly known as vitamin B3, is a precursor to nicotinamide adenine dinucleotide (NAD+) and nicotinamide adenine dinucleotide phosphate (NADP+), two of the most abundant coenzymes in our body.3 These coenzymes have chemical characteristics that allow them to rapidly transfer electrons between molecules and assist enzymes that typically perform redox, or electron-transfer, reactions.3
It is important to note that an enzyme can have more than one active site.3 Often, multiple active sites are used by enzymes which combine substrates, but sometimes they are needed to allow an enzyme to interact with multiple substrates simultaneously.3 One such example is transaminases, which catalyzes the movement of an amino group (NH4+) from one substrate to another in a manner that facilitates protein biosynthesis from amino acids. This molecular transfer is facilitated by the coenzyme pyridoxal phosphate (PLP), which is procured from vitamin B6.3
Mentioned above are just a few of the many fascinating coenzymes within the body, each with its own unique purpose. So, the next time you choose to ignore the vitamin or antioxidant label on the carton of orange juice and instead reach for a cup of coffee, think twice. The vitamins you are ignoring are in fact essential.
References
[1] Berg JM, Tymoczko JL, Stryer L. (2002). Biochemistry. 5th edition. New York: W H Freeman; Section 8.1, Enzymes Are Powerful and Highly Specific Catalysts; Section 8.6, Vitamins Are Often Precursors to Coenzymes. Available from: https://www.ncbi.nlm.nih.gov/books/NBK22380/
[2] Lehninger, A. L., Nelson, D. L., & Cox, M. M. (2000). Lehninger principles of biochemistry. New York: Worth Publishers.
[3] “Coenzymes.” Coenzymes – an Overview | ScienceDirect Topics, www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/coenzymes.
Related Posts
The Geography of Disease: a Bayesian Approach to Epidemiology
Figure 1: A map showing relative rates of pancreatic cancer...
Read MoreHow Genetic Engineering Can Responsibly Revolutionize Healthcare Treatment
Source: iStock At just 3 months old, Victoria Gray was...
Read MoreCOVID-19 Pneumonia – A Different Illness Than Its Traditional Counterpart
Figure 1: This is an image of a child with...
Read MoreAadhishre Kasat