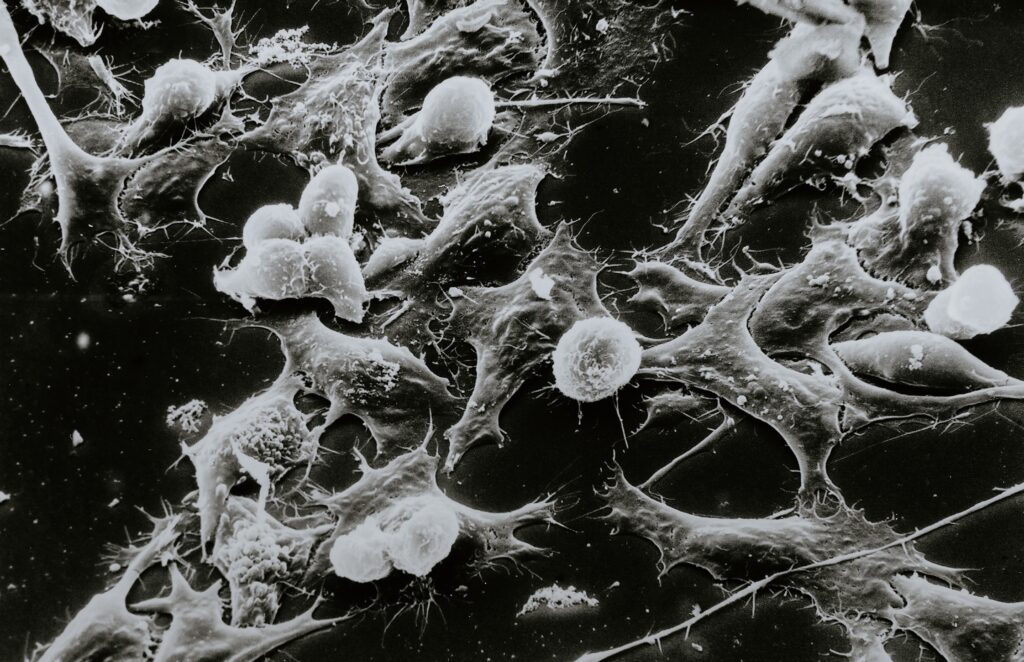
Figure 1: White Blood cells fighting tumor cells. (Source: National Cancer Institute)
Understanding Antibody-Dependent Cellular Cytotoxicity Mechanisms
Antibody-dependent cellular cytotoxicity (ADCC) is an immune response mechanism that harnesses the combined efforts of both the adaptive and innate arms of the immune system. The process involves several key components and steps.
- Antibodies as Mediators: ADCC begins with the binding of antibodies to antigens present on the surface of target cells. Antibodies are target-seeking proteins produced by certain immune cells, and their antigen targets can be part of a viruses, bacterium, or cancer cell. The work of antibodies is like finding bad cells and putting a big “X” on them. Once targeted by antibodies, other immune cells can join the assault.
- Fc Receptors on Effector Cells: The Fc (fragment crystallizable) region of the antibody binds to Fc receptors present on various other immune cells, particularly natural killer cells, macrophages, and neutrophils. These special immune cells are the effectors of ADCC and are used as backups to fight the tagged cells.
- Activation of Effector Cells: After binding to antibodies, effector cells become activated. Natural killer cells are particularly potent in ADCC due to their cytotoxic (cell-killing) abilities.
- Cellular Destruction: The activated immune cells release cytotoxic fragments, which contain substances like perfin and granzyme. These molecules initiate the apoptosis (cell death) of the target cell. Perfin (like the word perforate) creates holes in the target cell’s membrane, allowing granzymes to enter and induce apoptosis.
- Target Cell Lysis: Ultimately, with holes in the cell, the target cell undergoes lysis, leading to its destruction.
This process of ADCC ensures the elimination of infected or cancerous cells throughout the body1.
Therapeutic Potential of ADCC
Vaccine Development
ADCC has become a central focus in vaccine development strategies. Scientists are exploring ways to harness ADCC to create vaccines that are not only effective but also provide longer-lasting protection against a range of pathogens. For instance, HIV, the virus responsible for AIDS, has been particularly challenging to combat due to its ability to rapidly mutate and evade the immune system. Traditional vaccine approaches have struggled against HIV. However, researchers are developing vaccines that induce ADCC against HIV-infected cells. These vaccines aim to stimulate the immune system to produce antibodies that can recruit immune cells for ADCC, helping to clear HIV-infected cells from the body2. Similarly, ADCC is playing a crucial role in the development of vaccines against other infectious diseases like malaria and influenza3.
ADCC in Infectious Disease Treatment
Beyond vaccine development, ADCC is a key player in the treatment of established infections. Take, for example, COVID-19, caused by the SARS-CoV-2 virus. Antibody therapies like Regeneron’s REGEN-COV have been authorized for emergency use due to their ability to trigger ADCC. These antibodies are designed to bind to the spike protein of the virus, effectively tagging it for destruction by immune cells. This approach has shown promise in reducing the severity of COVID-19 in infected individuals4.
Transplantation
In the field of transplantation, ADCC presents a unique set of challenges and opportunities. Organ transplantation involves the transfer of organs from one person to another, which can trigger an immune response against the newly introduced organ (graft). ADCC plays a dual role here. Firstly, ADCC can be detrimental as it can contribute to graft rejection. Immune cells may recognize the transplanted organ as foreign and target it for destruction. To counteract this, researchers are exploring ways to suppress ADCC activity to prevent graft rejection, thereby improving the success rates of organ transplantation. By selectively targeting harmful immune cells, these therapies aim to preserve the transplant’s viability5.
Challenges Surrounding Antibody-Dependent Cellular Cytotoxicity
ADCC, despite its therapeutic potential, presents several challenges that warrant careful consideration. One significant challenge is the possibility of off-target effects, where therapeutic antibodies inadvertently bind to healthy cells, potentially causing unintended harm6. Furthermore, the risk of immunogenicity arises, as repeated use of antibodies can prompt immune responses against the therapeutic agents themselves, limiting their long-term effectiveness7. Additionally, the high cost of developing and administering ADCC-based therapies poses a barrier to access, raising concerns about healthcare disparities. There’s also the risk of overuse or misuse, especially if these therapies are perceived as a universal remedy8.
Ethical concerns accompany the promising applications of ADCC in medicine. Ensuring equitable access and affordability of ADCC-based therapies is crucial to prevent disparities in healthcare. Moreover, informed consent is paramount, necessitating clear and comprehensive patient education about the potential risks and benefits of these treatments. Profit motives can overshadow patient welfare, leading to questions about the ethical implications of commercialization and intellectual property control in this field. Unintended consequences, such as the promotion of pathogen resistance, raise ethical dilemmas concerning patient safety and public health. As ADCC therapies continue to advance, addressing these ethical concerns and developing appropriate regulatory frameworks becomes imperative to safeguard both individual and societal interests9.
Conclusion
Targeting and manipulating the antibody-dependent cellular cytotoxicity (ADCC) response represents a promising avenue in healthcare, particularly in vaccine development, infectious disease treatment, and transplantation. As seen in the fight against HIV and COVID-19, as well as efforts to improve organ transplantation, ADCC-based strategies are advancing, offering hope for more effective vaccines, better treatment options, and improved outcomes in these critical areas of medicine.
References
- Surat, P. (2023). Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC). Medical News. https://www.news-medical.net/life-sciences/Antibody-Dependent-Cell-Mediated-Cytotoxicity-(ADCC).aspx
- Yaffe et al. (2023). Reconstruction of a polyclonal ADCC antibody repertoire from an HIV-1 non-transmitting mother. iScience 26 (5): 106762
- Opi et al. (2021). Multi-functional antibody profiling for malaria vaccine development and evaluation. Expert Review of Vaccines 10: 1257-1272
- Yu et al. (2021). Antibody-dependent cellular cytotoxicity response to SARS-CoV-2 in COVID-19 patients. Signal Transduction and Targeted Therapy 6: 346
- Legris et al. (2016). Antibody-Dependent NK Cell Activation Is Associated with Late Kidney Allograft Dysfunction and the Complement-Independent Alloreactive Potential of Donor-Specific Antibodies. Frontiers in Immunology 7: 288
- Nejadmoghaddam et al. (2019). Antibody-Drug Conjugates: Possibilities and Challenges. Avicenna Journal of Medical Biotechnology 11(1): 3-23
- Munagala et al. (2014). Alternate approaches addressing variability in ADCC assay. Journal of Bioanalysis & Biomedicine.
- Lewis et al. (2019). Knowns and Unknowns of Assaying Antibody-Dependent Cell-Mediated Cytotoxicity Against HIV-1. Frontiers in Immunology 10: 1025
- Schofield, Desmond. (2022). Monoclonal antibodies – all information. Evitria. https://www.evitria.com/journal/monoclonal-antibodies/monoclonal-antibodies/
Related Posts
Caribbean History through Genetics and Archaeology
Figure 1: A map of the Caribbean Islands from 1894...
Read MoreThe Public Health Humanitarian Crisis in Ukraine
This publication is in proud partnership with Project UNITY’s Catalyst Academy 2023...
Read MoreThermophilic Life in Yellowstone National Park Challenges the Physical and Chemical Limits of Survival
Cover Image: Grand Prismatic Spring in Yellowstone National Park (Source:...
Read MoreHow the Bystander Effect on Security Robots can Hamper Law Enforcement
BigDog robots being tested under the supervision of a Boston...
Read MoreChildhood Unpopularity May Increase Risk of Cardiovascular Disease in Adulthood
Figure 1: Childhood experiences are widely considered to affect adult...
Read MoreIntergenerational Trauma: How a history of pain brings biological costs
Source: Nicolas Raymond, CC BY 2.0 A few years ago,...
Read MoreAnisha Shukla