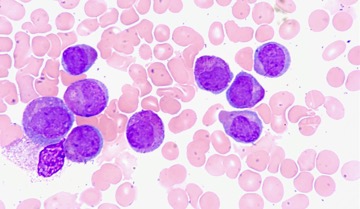
Figure 1: The image is taken from an elderly woman who has a type of acute myeloid leukemia in which the accumulation of immature blood-forming cells (purple) interferes with the production of normal white blood cells (pink)
Source: Wikimedia Commons
Leukemia is a type of blood cancer generally characterized by the suppression of proper function in white blood cells (Mayo Clinic, 2021). Acute myeloid leukemia (AML) is a subtype in which this process is induced by an uncontrolled production of immature white blood cells in the bone marrow, leading to rapid disease (American Cancer Society, 2021). One cell central to the immune response against cancer is the T lymphocyte, which is vital to adaptive immunity and the coordinated killing of cells expressing certain molecules, called antigens (Liu & Peng, 2021). The ability to selectively recruit specific types of T cells for the immune response against leukemia cancer cells could prove helpful toward the development of novel immunotherapies (Ganesan et al., 2021).
Rajkumar Ganesan et al. of Johnson & Johnson set out to determine whether further investigating the area would be helpful. The researchers engineered an antibody—or component proteins that specifically recognize the antigens on cells that the immune system is aiming to mount a response against- capable of simultaneously binding to both the receptor of Vγ9+ γδ T cells, or γδ T cells positive for the Vγ9+ variable chain in their receptor, and CD123, a target molecule that serves as a marker for leukemia cells (Ganesan et al., 2021). Essentially, the idea behind this bispecific antibody is that it could efficiently mediate an interaction between a killer cell and the cell it is killing, since it is able to bind both. Clinical trials have shown that leukemia cell-targeting by another type of T cell, pan CD3+, proves to be largely ineffective and often is toxic to patients (Benmebarek et al., 2021). Accordingly, these scientists decided to explore Vγ9+ γδ T cells instead. They hypothesized that sucessful recruitment of Vγ9+ γδ T cells could direct higher rates of binding to- and hopefully killing of- cancer cells.
Fortunately, the engineered bispecific antibody successfully activates Vγ9+ γδ killer T cells. Researchers discovered this by culturing two groups of T cells in the presence of antibody; ideally, the Vγ9+ γδ cells would be activated, and Vγ9- cells, as well as CD4+ and CD8+ αβ cells, would serve as a negative control. They tracked four molecular markers for activated T cells: CD69, CD25, surface expression of CD71, and intracellular expression of Granzyme B. Results showed that there was indeed a very significant difference in expression levels of these markers between the two categories of T cells, indicating that the Vγ9+ γδ/CD123 antibody successfully induces Vγ9+ γδ T cells to enter their activated state (Ganesan et al., 2021). In other words, the scientists determined that the engineered antibody activates the T cell subtype of interest by culturing those T cells in the presence of antibody and finding that certain molecular markers indicative of activation were expressed as a result.
Cell-cell conjugation is important because it serves as a prerequisite for immune cell killing; adhesion and co-stimulatory molecules result in the formation of an “immunological synapse” (Dustin, 2015). This immunological synapse refers to a stable interaction between the T cell and the cancer cell it is recognizing, and is necessary for T cell-mediated killing to occur (Huppa & Davis, 2003). Researchers used a similar method as described earlier to explore whether the engineered bispecific antibody effectively led to conjugation of Vγ9+ γδ T cells to Kasumi-3 tumor cells. These cells were cultured together in the presence of two different bispecific antibodies, one being the antibody of interest and the other serving as a negative control. Results showed successful conjugation activity in the presence of Vγ9+ γδ/CD123 antibody, suggesting it was able to induce the immunological synapse necessary for the T cell-mediated response (Ganesan et al., 2021). In short, culturing T cells and cancer cells together in the presence of engineered antibody revealed that the interaction necessary for cancer cell death was accomplished by the antibody.
Perhaps the study’s most promising result was the demonstrated efficient Vγ9+ γδ T cell-mediated killing of target cells. T cells were cultured together with three types of target cells in the presence of varying concentrations of bispecific antibody. Cytotoxicity was determined by measuring target cell lysis (i.e., bursting open) at the given concentration. The researchers also accounted for spontaneous cytotoxicity of target cells by measuring their lysis when cultured without presence of antibody or effector T cells as a control. Results concluded that cytotoxicity was successfully mediated and dose-dependent, with about 6% death of Kasumi-3 cells for 0.01 ng/mL of antibody and 65% death of Kasumi-3 cells for 1 ng/mL of antibody (Ganesan et al., 2021).
This research shows the potential for treatment of acute myeloid leukemia by targeting blasts with Vγ9+ γδ T cells using an engineered bispecific antibody for their selective recruitment, as well as efficient conjugation with and cytotoxic protein-mediated killing of tumor cells. Taken together, the results suggest there could be major promise in further investigating whether this strategy has a role to play in immunotherapies.
References
Benmebarek, M., Cadilha, B., Herrmann, M., Lesch, S., Schmitt, S., Stoiber, S., . . . Kobold, S. (2021). A modular and controllable t cell therapy platform for acute myeloid leukemia. Retrieved February 22, 2021, from https://www.nature.com/articles/s41375-020-01109-w
Dustin, M. (2014). The immunological synapse. Retrieved February 22, 2021, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4692051/
Ganesan, R., Chennupati, V., Ramachandran, B., Hansen, M., Singh, S., & Grewal, I. (2021). Selective recruitment of γδ t cells by a bispecific antibody for the treatment of acute myeloid leukemia. Retrieved February 20, 2021, from https://www.nature.com/articles/s41375-021-01122-7
Huppa, J., & Davis, M. (2003). T-cell-antigen recognition and the immunological synapse. Retrieved February 23, 2021, from https://www.nature.com/articles/nri1245
“Leukemia.” (2021). Mayo Clinic. Retrieved February 20, 2021, from https://www.mayoclinic.org/diseases-conditions/leukemia/symptoms-causes/syc-20374373
Liu, X., & Peng, G. (2021). Mitochondria orchestrate t cell fate and function. Retrieved February 22, 2021, from https://www.nature.com/articles/s41590-020-00861-6
“What is acute myeloid leukemia (aml)?: What is aml?” (2021). American Cancer Society. Retrieved February 20, 2021, from https://www.cancer.org/cancer/acute-myeloid-leukemia/about/what-is-aml.html
Related Posts
UV-Vis Spectroscopy: A Step in the Light Direction
Executive Board Advisor: Nishi Jain Co-Authors: Daniela Galvez-Cepeda, Katherine Faulkner,...
Read MoreGenomic Research For All Humanity
From DNA to Life. (Source: Wikimedia Commons, William Crochot) Our...
Read MoreThe Next Source of Antibiotics: Viruses
Figure 1: A computer-generated image of a bacteriophage called a...
Read MoreMatthew Lutchko


Comments are closed.