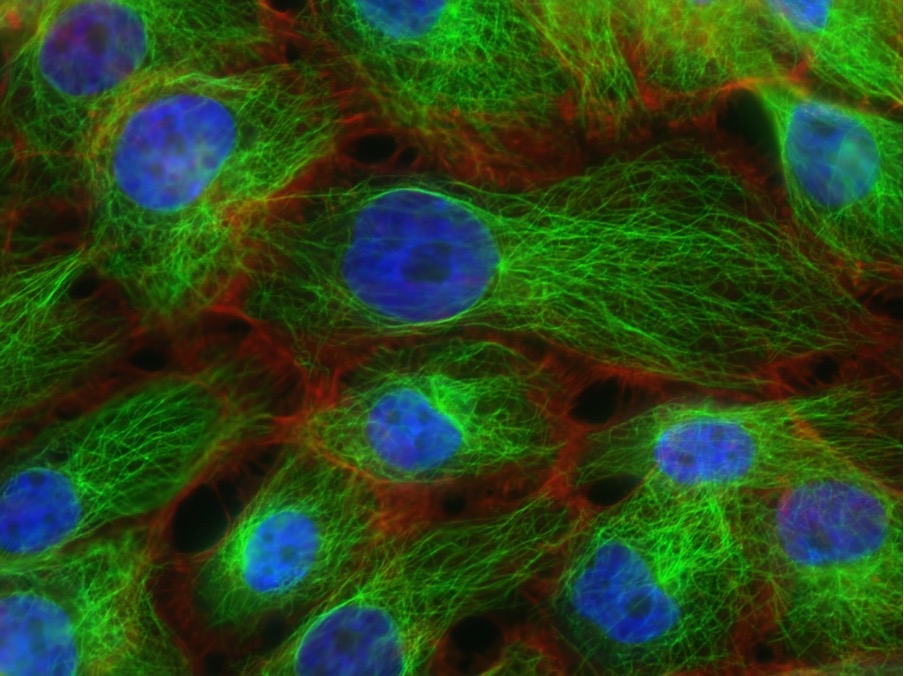
Figure 1: This image displays microtubules – green fibre-like structures of the cell’s cytoskeleton – in human breast cancer tissue. Immunotherapy is a form of cancer treatment that induces the immune system to fight back against tumors. Although it has been a successful form of therapy, there are still several challenges that limit the effectiveness of the treatment.
Source: Unsplash (National Cancer Institute)
Cancer is one of the most infamously destructive diseases that plagues our world. The key to its fatal perseverance is that cancerous cells, however mutant, are still human ‘self’ cells that our immune systems are trained not to fight. One popular method of treatment, called immunotherapy, utilises immune cells, specifically T-cells, to target certain surface proteins which are overexpressed on tumour cells. T-cell immunotherapy works by obtaining the natural T-cells from a patient, engineering them to express chimeric antigen receptors (CAR) that target proteins on the surface of cancer cells, and infusing them back into the patient to begin attacking the cancer. Although this form of therapy is very beneficial, one issue is that the same target antigens can be found at low levels on healthy tissue. These CARs usually target receptors such as epidermal growth factor receptors (EGFRs) or human epidermal growth factor receptor 2 (HER2s), which are ubiquitous on many cell types, resulting in non-discriminatory cytotoxic activity against any cell displaying them. This can lead to off-target tumour killings, which can be lethal for the patients in some cases (Morgan et al., 2010).
A 2021 study published in Science Magazine looked to overcome this major issue by employing a novel anti-cancer T-cell activation procedure (Hernandez-Lopez et al., 2021). Previously, T-cells have been designed to constitutively display CARs on the cell surface, whereas in this new design, getting the T-cells to express CAR is a two-step process. The new circuit utilises a positive feedback loop whereby the antigen – in this case, HER2 — is first detected by low-affinity synthetic Notch (synNotch) receptors, which then induce the expression of CARs against the same antigen in T-cells if the threshold of antigen density is fulfilled – essentially, turning on the switch for T-cell activation only when there is high antigen density. Thus, the products of the initial interaction amplify the interaction even further and are not switched on against healthy cells with low antigen density. The synNotch receptors are modified versions of the normal human Notch receptor, which is constructed by fusing anti-HER2 single-chain Fv (scFV) regions with mouse Notch regulatory domain, along with the other necessary components needed for the complete receptor. SynNotch technology has become commonly used for customized cell sensing for antigens of interest, because of their direct and simple mechanism of signalling (Morsut et al., 2016). The binding of the antigen to synNotch receptors both initiates the activation of the T-cells and increases the sensitivity of them to HER2, through this circuit of positive feedback.
The researchers experimented on a series of stable human leukaemia tumour cells with the cells expressing a variety of different HER2 densities over a 100-fold range. The goal was for the novel T-cells to be able to distinguish between HER2-amplified cancer cells that express greater than 106.5 molecules per cell from normal HER2-expressing cells that express around 104.5 molecules per cell.
Hernandez-Lopez et al. found that the novel T-cells were able to discriminate between these two different HER2 expressions, exhibiting this ultrasensitive threshold, whereas the conventional T-cells did not. In addition, several different synNotch receptors and CAR combinations were tested with the affinity of the receptors to HER2 spanning a 100-fold range. The most effective circuit was shown to have a low-affinity synNotch receptor controlling the expression of a high-affinity CAR.
The results of this novel immunotherapy strategy are promising, with effective discrimination between high and low antigen-density cells found in vitro and in vivo. Other challenges, such as tumour micro-environments and tumour heterogeneity, are still considerable obstacles to the efficacy of the treatment which can severely dampen the infiltration, activation and effect of the T-cells (Zhang et al., 2016). For instance, the tumour microenvironment can downregulate surface antigens that are necessary for T-cells recognition, or can generate immunosuppressive mechanisms, such as releasing IL-10, that extinguish T-cell attack (Alireza Labani Motlagh, 2020). Research to extend this two-step circuit to other target antigens, like EGFRs, that are over-expressed in cancers, as well as to other types of cancers, will inevitably be required in the future. Nevertheless, Hernandez-Lopez et al. have certainly shown that using smart, sophisticated design is essential for overcoming and improving anti-cancer immunotherapies for the better.
References
Labani-Motlagh A, Ashja-Mahdavi M and Loskog A (2020) The Tumor Microenvironment: A Milieu Hindering and Obstructing Antitumor Immune Responses. Front. Immunol. 11:940. doi: 10.3389/fimmu.2020.00940
Hernandez-Lopez, R. A., Yu, W., Cabral, K. A., Creasey, O. A., Lopez Pazmino, M. D. P., Tonai, Y., . . . Lim, W. A. (2021). T cell circuits that sense antigen density with an ultrasensitive threshold. Science, 371(6534), 1166-1171. doi:10.1126/science.abc1855
Morgan, R. A., Yang, J. C., Kitano, M., Dudley, M. E., Laurencot, C. M., & Rosenberg, S. A. (2010). Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther, 18(4), 843-851. doi:10.1038/mt.2010.24
Morsut, L., Roybal, K. T., Xiong, X., Gordley, R. M., Coyle, S. M., Thomson, M., & Lim, W. A. (2016). Engineering Customized Cell Sensing and Response Behaviors Using Synthetic Notch Receptors. Cell, 164(4), 780-791. doi:10.1016/j.cell.2016.01.012
Zhang, B. L., Qin, D. Y., Mo, Z. M., Li, Y., Wei, W., Wang, Y. S., . . . Wei, Y. Q. (2016). Hurdles of CAR-T cell-based cancer immunotherapy directed against solid tumors. Sci China Life Sci, 59(4), 340-348. doi:10.1007/s11427-016-5027-4
Related Posts
Beyond the White Coat Worker: An Overview of Citizen Science
By: Olivia Brado1, Luz Mariana Cumpa Gomez1, Shirin Dadina1, Katrina...
Read MoreDopamine Deficiency and Its Effects on Addiction
This publication is in proud partnership with Project UNITY’s Catalyst Academy 2023...
Read MoreFighting Hospital Hardships: An Intervention for Reducing Stress in Hospital Workers
Figure 1: This figure describes some of the symptoms of...
Read MoreLilly Tozer