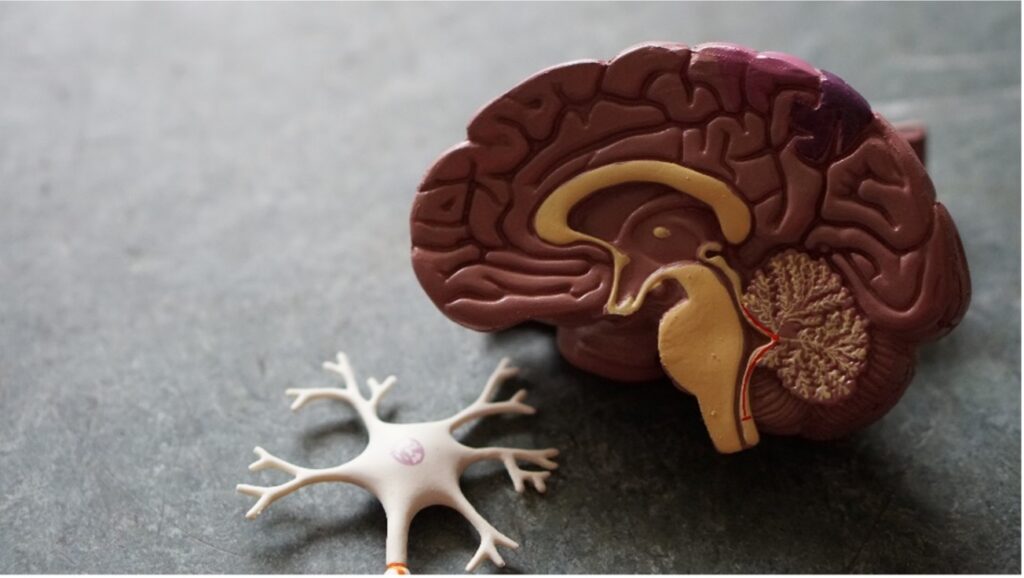
Figure 1: A cross section of a model brain
Source: unsplash.com
Alzheimer’s Disease (AD) is a progressive, irreversible neurological condition that slowly destroys memory and cognitive skills and can be classified as either early or late onset. Most people diagnosed with Alzheimer’s develop symptoms in their mid-60s (late onset), whilst early onset Alzheimer’s is much rarer and symptoms can occur as early as 30 years old (NIH, 2021). In older adults, AD is the most common form of dementia, the loss of cognitive functioning and behavioural abilities to the extent that it interferes with the daily life; the disease accounts for 60-80% of dementia cases, a huge fraction of the 47 million people living with dementia globally. This latter number is expected to triple by 2050 as the population ages and life expectancy continues to increase (Tiwari, 2019).
A vital protein involved in AD is amyloid beta, a naturally occurring protein in healthy brains that accumulates excessively and forms toxic plaques between the brain cells of those with AD. Another characteristic of Alzheimer’s is the build-up of toxic tau proteins. Tau proteins help to regulate brain function, but in excess tau protein tangles and toxic plaques cause brain-cell death and symptoms including memory loss and progressive neurocognitive decline. The damage originates in the hippocampus, the area of the brain that is essential for memory formation. As the disease progresses and neurones continue to die, additional parts of the brain are affected and the brain tissue significantly shrinks in size (NIH, 2021).
The FDA has not approved a drug treatment for AD since the NMDA receptor agonist Memantine was approved in 2004. Memantine blocks the neurotransmitter glutamate, a neurotransmitter that is typically present in higher levels in those with AD. This causes too much calcium to move into the brain cells, resulting in cell death. Memantine binds to glutamate receptors to prevent excess calcium update, alleviating some cognitive functionality. Currently there is no available drug that slows the progression, only medication that lessens symptoms such as memantine (Johnson, 2006). However, Biogen has recently developed the monoclonal antibody Aducanumab that has been proven to slow patients cognitive and functional decline. Antibodies perform two main functions, recognise a target protein, or antigen, on target cells, and trigger an immune response. Aducanumab is engineered to bind to antigens present on aggregated amyloid beta plaques and trigger an immune response to destroy them (Biogen , 2021). This drug has the potential to change Alzheimer’s from a fatal disease to one that is manageable with the correct prescription, like HIV/AIDs can be managed today.
Biogen carried out two simultaneous trials with different dose strengths compared with placebo over 18 months. These trials were halted at first in March 2019 due to lack of success, but after subsequent analyses using 3 months of extra data Biogen claimed efficacy and applied for FDA approval. These analyses showed the trial with the higher dose (10mg/kg) had a 40% slower rate of decline and less amyloid and tau protein build-up compared with placebo. Subjects on the lower dose (1mg/kg) showed no significant slowing of cognitive decline. Although this is a massive break-through in Alzheimer treatment, there are some shortcomings. Firstly, there is insufficient data to prove efficacy and further trials are needed (Knopman, 2020). Secondly, Aducanumab needs to be administered by IV infusion every week until death, costing $50,000 per patient per year. This is vastly more expensive than memantine that is delivered orally, costing only $6700 per patient per year (Knapp, 2017). Despite these concerns, research is closer to a cure than ever before. The future for Alzheimer’s therapy is bright.
References
Biogen . (2021, January 29). News Releases. Retrieved from Biogen: https://investors.biogen.com/news-releases/news-release-details/biogen-and-eisai-announce-fdas-3-month-extension-review-period
Budson, A. E. (2020, November 12). A new Alzheimer’s drug: From advisory panel to FDA -what’s at stake here? Retrieved from Harvard Health Publishing : https://www.health.harvard.edu/blog/a-new-alzheimers-drug-from-advisory-panel-to-fda-whats-at-stake-here-2020111221380#:~:text=Cost%20also%20needs%20to%20be,impairment%20and%20mild%20dementia%20stages.
Johnson, J. (2006). Mechanism of action of memantine. Current Opinion in Pharmacology, 61-67.
NIH. (2021, March 20). What is Alzheimer’s Disease? Retrieved from National Institute on Ageing : https://www.nia.nih.gov/health/what-alzheimers-disease#:~:text=Alzheimer’s%20disease%20is%20an%20irreversible,appear%20in%20their%20mid%2D60s.
Robertson, S. (2018, August 23). What are Amyloid Plaques. Retrieved from Medical Life Sciences News: https://www.news-medical.net/health/What-are-Amyloid-Plaques.aspx
Tiwari, S. (2019). Alzheimer’s disease: pathogenesis, diagnostics and therapeutics . International Journal of Nanomedicine , 5541-5554.
Williams, S. (2019). Biogen Presents Data on Efficacy of Alzheimer’s Drug . The Scientist .
Related Posts
Dopamine Deficiency and Its Effects on Addiction
This publication is in proud partnership with Project UNITY’s Catalyst Academy 2023...
Read MoreCOVID-19 Clinical Trials and Racial Disproportionality
Figure 1: A doctor drawing blood from a patient as...
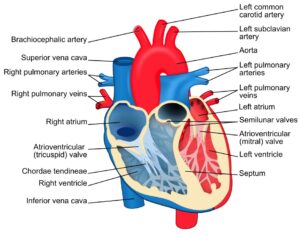
Read MoreAn International Team of Researchers Just Solved One of the Biggest Mysteries About the Human Heart
Figure 1: The heart is one of the most complex...
Read MoreAmber Stiby