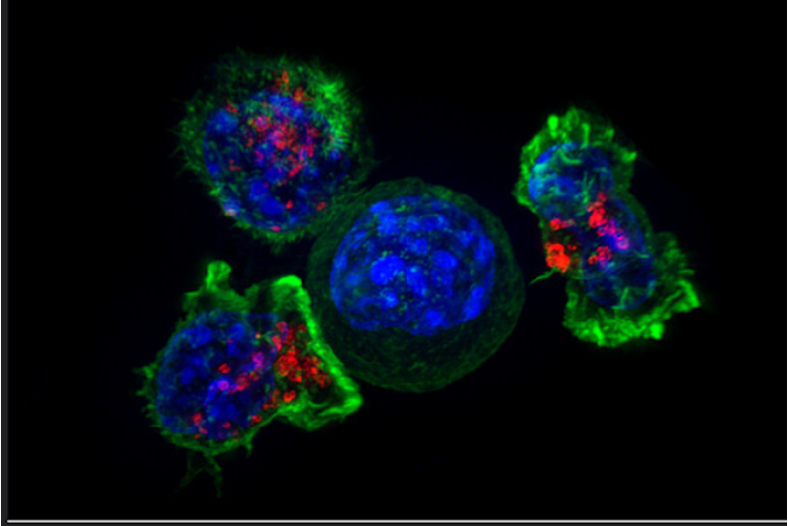
Figure 1: T cells (green, blue, and red) surround and prepare to kill a cancer cell (green and blue). Vesicles (shown in red) in the T cells contain perforin and granzymes, proteins that orchestrate the killing of target cells. This process of targeted cell destruction is compromised when mitochondria are rendered defective by excessive antigen stimulation from cancer cells
Source: Wikimedia Commons
The T lymphocyte, a type of white blood cell vital to the adaptive immune response and coordinated killing of specific pathogens, is a key player in anti-cancer immunity (Kumar et al., 2019). Recent research exploring the mechanisms of T cell stress and exhaustion in the tumor microenvironment has shed light on the causative relationship between mitochondrial defects and T cell dysfunction (Liu & Peng, 2021).
Nichole Scharping at the University of Pittsburgh found that terminally exhausted T cells, “defined as expressing high levels of programmed cell death protein 1 (PD-1),” experienced elevated levels of hypoxia relative to other T cells. Cellular hypoxia refers to a low-oxygen state, typically resulting from an environment with limited oxygen supply (Samarinas & Gravas, 2018). To determine whether hypoxia has the direct ability to cause this exhausted state in T cells, Scharping and her team conducted experiments exposing T cells to hypoxic conditions. They found that T cells were able to withstand solely hypoxic conditions, but were brought to their terminally exhausted state when conditions of hypoxia were combined with continuous stimulation of T cell receptors by tumor cell antigens. Subsequent findings from this study demonstrate that the dysfunctional state is largely caused by mitochondrial changes within the T cells (Scharping et al., 2021).
Under these stimulatory and hypoxic conditions, Scharping found the transcriptional repressor B lymphocyte-induced maturation protein-1 (Blimp-1) to be expressed at higher levels. Previous research indicated that T cells lacking Blimp-1 were unable to achieve a terminally exhausted state. Scharping discovered that upregulation of Blimp-1 led to the inhibition of PGC-1α, a coactivator with important roles in increased mitochondrial mass, or mitochondrial biogenesis (Liang & Ward, 2006). Considering that failure to induce biogenesis is associated with molecular issues, including lower cisternae surface area and thus lower rates of oxidative phosphorylation, knowledge of this pathway indicated that continuous antigen stimulation under hypoxic conditions was sufficient in affecting mitochondrial activities (Scharping et al., 2021). In particular, overexpression of PGC-1α leads to the transcription of mitochondrial genes involved in preventing a terminally exhausted state for the T cell. Clearly, loss of function in PGC-1α corresponds to a loss in translated genes that inhibit an exhausted-like dysfunctional state.
Since reactive oxygen species (ROS) can be promoted by mitochondrial dysfunction and are more highly produced under continuous antigen stimulating conditions, the researchers decided to investigate if and how ROS plays a major role in driving the exhaustion of T cells (Scharping et al., 2021).
The ‘if’ portion of this question was answered by activating and growing T cells in the presence of antimycin A, a mitochondrial complex III inhibitor that causes the production of ROS. Twenty-four hours later, cellular markers indicated these T cells were induced to a terminally exhausted-like state, suggesting that ROS presence does in fact cause T cell exhaustion. ROS and their byproducts are known to inhibit enzymes that remove phosphates from tyrosine. Signaling pathways in T cells heavily involve tyrosine phosphorylation mechanisms, so the researchers reasoned ROS may be interfering with them by locking the pathways in an activated state. Indeed, they found that increased ROS levels drove persistent NFAT1 signaling, a “transcriptional circuit known to drive exhaustion.” At this point, the scientists had learned that continuous antigen stimulation under hypoxic conditions caused mitochondrial reprogramming events which supported an increase in ROS levels, ultimately driving T cells to their exhausted state (Scharping et al., 2021).
Notably, the researchers found that successful mitigation of hypoxic conditions as well as a reduction in ROS production both allowed for relief from T cell exhaustion. Previous data also found that “reversing the loss of PGC1α in tumor-specific T cells results in superior antitumor immune response” (Scharping et al., 2021). These facts, taken in combination with other learned details and the future study of these and related mechanisms, could have significant implications for cancer immunotherapy. Preventing T cell exhaustion might allow the immune system to fight back against rogue cancer cells.
References
Kumar, B., Connors, T., & Farber, D. (2018). Human t cell development,
localization, and function throughout life. Retrieved February 16, 2021, from
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5826622
Liang, H., & Ward, W. F. (2006). Pgc-1alpha: A key regulator of energy metabolism. Retrieved February 16, 2021, from https://pubmed.ncbi.nlm.nih.gov/17108241/
Liu, X., & Peng, G. (2021). Mitochondria orchestrate t cell fate and function. Retrieved February 16, 2021, from https://www.nature.com/articles/s41590-020-00861-6
Samarinas, M., & Gravas, S. (2018). The relationship between inflammation and luts/bph. Retrieved February 16, 2021, from https://www.sciencedirect.com/science/article/pii/B9780128113974000032
Scharping, N. E., Rivadeneira, D. B., Menk, A. V., Vignali, P. D., Ford, B. R., Rittenhouse, N. L., . . . Delgoffe, G. M. (n.d.). Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives t cell exhaustion. Retrieved February 16, 2021, from https://pubmed.ncbi.nlm.nih.gov/33398183/
Related Posts
When the Brain Loses a Sense, How Does it Compensate?
Source: Wendel Moretti The brain plays a crucial role in...
Read MoreGender Differences in Health and Medicine and the Importance of Gender-Specific Medicine in Healthcare Policy
This article was originally submitted to the Modern MD competition...
Read MoreVaping and Mental Fog – The Connection Between E-Cigarette Use and Mental Function
Figure 1: Use of e-cigarette devices, like the one in...
Read MoreMatthew Lutchko