First Author: Jillian Troth1
Co-Authors [Alphabetical Order]: Caroline Conway1, Allison Kifer2, Vincent Lai4, Grace Lee3, Varun Lindagal1, Callie Moody1, and Melanie Prakash1
Dartmouth College1, University of California San Diego2, Williams College3, Rice University4
Figure 1: Fluorescent microscope imaging of neuroinflammation in wild-type mice, AD-model mice, and AD patient. Aggregation of amyloid precursor protein (red) indicates inflammation and precedes formation of Aβ (green), a protein characteristic to AD. AD = Alzheimer’s Disease
Source: Wikimedia Commons
Neurodegenerative diseases (NDs) pose a growing threat to global health. In 2016, Alzheimer’s Disease (AD) afflicted 5.4 million Americans, and Parkinson’s Disease (PD) currently impacts 10 million people worldwide (National Institute of Environmental Health Sciences, 2019; Parkinson’s Foundation, n.d.). As modern medicine extends average life expectancy, the burden of these age-associated diseases will only increase. By 2060, it is expected that 1 in 45 Americans and 1 in 85 people globally will be diagnosed with a ND; 14 million people in the US will likely be living with AD at that point in time (Gao et al., 2019; Division of Population Health, 2020). Without a cure or effective treatment for neurodegenerative disorders, the medical field is faced with a daunting challenge. Thus, it is imperative to establish a firm understanding about the pathogenesis of NDs.
Theories of ND Pathogenesis
NDs are primarily characterized by a progressive loss of neurons and are classified by their clinical features, the anatomy of neurodegeneration, or molecular abnormalities. There are fundamental processes associated with progressive neuronal dysfunction and death shared amongst NDs. The most common molecular pathophysiologies of NDs are amyloidoses, tauopathies, α-synucleinopathies, and transactivation response DNA binding protein 43 (TDP-43) proteinopathies (Dugger and Dickson, 2017). Amyloidoses are NDs in which patients present with particularly high intra- or extracellular accumulations of amyloid, an insoluble fibrous protein, most commonly in the form of amyloid-β (Aβ) or prion protein (PrP) (Dugger and Dickson, 2017). Similarly, tauopathy patients present with aggregations of tau protein, α-synucleinopathies with presynaptic protein α-synuclein, and TDP-43 proteinopathies with TDP-43 (a multifunctional nuclear protein that can locate extracellularly in NDs) (Dugger and Dickson, 2017).
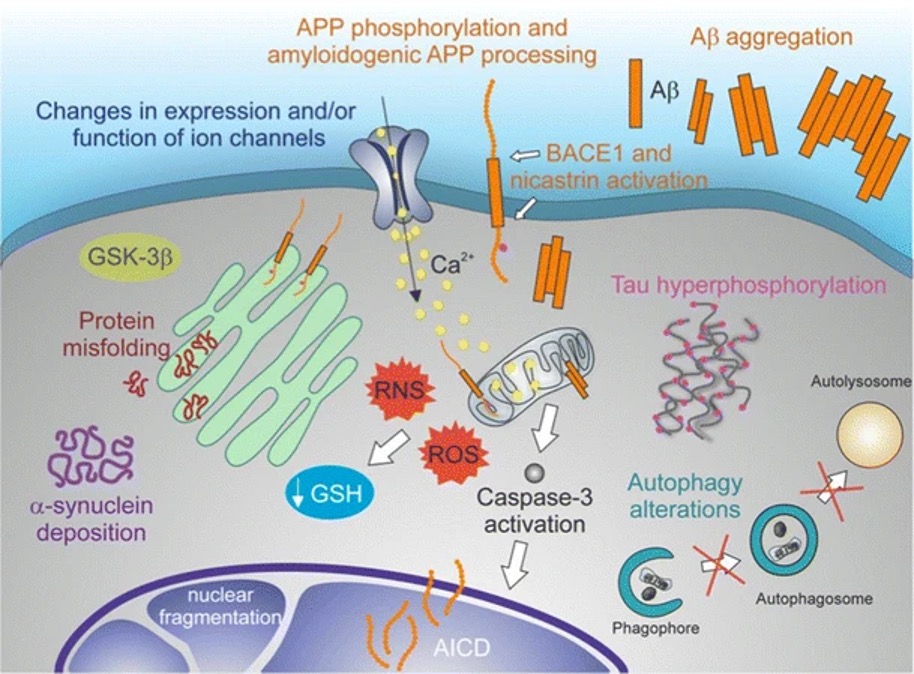
Figure 2: A molecular model of neurodegeneration. Common etiological features of NDs include amyloidoses, tauopathies, α-synucleinopathies, and TDP-43 proteinopathies
Source: Wikimedia Commons
NDs are thought to result from a wide variety of genetic and environmental factors. In determining which factors may cause NDs to occur, it is important to understand that while NDs are typically diagnosed at age 50 or older, neurobiologists have found that the causal processes begin twenty to thirty years before the first pathological symptoms are recognized and diagnosed (Castillo et al., 2019).
There are four major proposed routes regarding this gradual etiology of NDs. These include vascular origin, cellular senescence, body-brain trophic interactions (nutrition), and gut dysbiosis, the last of which is the major focus of this paper (Castillo et. al, 2019). According to the vascular origin theory, NDs may arise secondary to vascular diseases (such as hypertension, atherosclerosis, cerebral small-vessel disease, and atrial fibrillation) when cerebrovascular functioning is consequently compromised, failing to maintain homeostasis in the brain and thereby altering neural activity (Castillo et al., 2019). Broadly, the cellular senescence theory posits that when cells age, they become inflamed and local cell senescence (the inability to replicate) is brought on by the activation of various cell signaling pathways (Castillo et al., 2019). The brain-body trophic interactions theory of neurodegeneration focuses on the effects of alterations to parts of the peripheral nervous system on their corresponding parts of the central nervous system (Castillo et al., 2019). Finally, the gut dysbiosis theory says that pathological alterations to the gut microbiota manifest in systemic inflammation and circulation of neural toxins, thereby disrupting neurological function (Castillo et al., 2019).
The gut-microbiota hypothesis of neurodegeneration
Although the human body is often seen as a singular entity, it is home to a vast array of microorganisms inhabiting different areas of the body. The bacteria, archaebacteria, and viruses that live in the gut are collectively referred to as the gut microbiota and account for over 90% of living cells found there (Sandoval-Motta et al., 2017). While the gut is primarily a digestive organ, it also is the largest immune and endocrine organ in the body and has its own branch of the nervous system called the enteric nervous system (ENS) which is distinct from the brain/central nervous system (CNS) (Liddle, R. A., 2018). Notably, the ENS has the potential to influence the CNS, suggesting that some neuropathologies may begin in the gut. The functionality of these independent pathways is dictated by the cooperative relationship between the gut and its microbiome (Forsythe et al., 2010). Thus, according to the “gut-microbiota” hypothesis for disease pathogenesis, exposure to bodily stress, antibiotic intake, and/or an unhealthy diet might disturb the gut microbiota’s important functions and thereby contribute to various neurological diseases (Liang et al., 2018a).
In support of this gut-microbiota hypothesis, a series of experiments performed by the Key Laboratory of Mental Health at the Chinese Academy of Sciences found that a fat-saturated diet, use of antibiotics, and gastrointestinal disease are all associated with gut microbiota abnormalities in rats and resulted in anxiety and depression. After introducing therapeutic probiotic bacterial strains to these rats, the aforementioned symptoms significantly improved (Wang et al., 2015; Luo et al., 2014; Hu et al., 2014). This experiment is just one of many that support the gut-microbiota hypothesis for disease pathogenesis and suggests that alterations to one’s lifestyle, through actions like the inclusion of probiotics and an improved diet, will have beneficial effects on neurological health (Liang et al., 2018b).
Gut dysbiosis, which at the most basic level refers to a change in the composition and function of the bacterial community residing in an individual’s gut, has been implicated in the development of several chronic diseases which can increase likelihood of developing NDs later in life (Levy et al., 2017; Castillo et al., 2019). One example of this is metabolic disorder of the gut, which is considered a risk factor for AD and other NDs. It is thought that metabolic alterations in the gut can induce low-grade chronic inflammation that causes NDs. Furthermore, the permeability of the gut epithelium increases with age and may allow bacteria and their bacterial products to translocate to other parts of the body including the brain. Though further studies are required, it is believed that these translocated bacteria may cause further neurodegeneration by producing an abnormal immune response. While countless other factors lead to ND development as well (including hypertension, atrial fibrillation, systemic metabolic disease, and diabetes), recent research has demonstrated well the potential causative role the gut plays in affecting brain function (Castillo et al., 2019).
This paper will review major mechanisms of gut-brain communication and synthesize evidence in support of the gut-microbiota hypothesis of ND pathogenesis. Specifically, it will explore the links between MGBA dysfunction and AD and PD etiology, respectively. By highlighting the evidence for a gut-brain theory of ND pathogenesis, we hope to spread awareness about the promise of MGBA research for explaining the origins of these diseases. Furthermore, we wish to encourage further dedication of resources towards pursuing a better understanding of the MGBA as it relates to NDs.
The Microbiota-Gut-Brain Axis
Pathways of MGBA communication
The microbiota-gut-brain axis (MGBA) is a multidirectional system of nervous, endocrine, and immune pathways (Shan et al., 2018). The MGBA serves as a mechanism through which the gut microbiome can regulate central nervous system function. The central, autonomic, and enteric nervous systems all contribute to MGBA activity (Dempsey et al., 2019). In top-down regulation of the MGBA, brain signaling can regulate the gastrointestinal tract’s sensory, secretory, and motor activity through vagus nerve fibers (Ronan et al., 2021). As for bottom-up processing in the MGBA, in which the gut microbiota regulates central nervous system activity, Dempsey et al. identify several key mechanisms including bacteria-driven chemical alteration of neurotoxicants, stressor-induced changes in metabolite production, and change in intestinal barrier integrity (2019).
A neurotoxicant is any substance which adversely affects the function of the central and/or peripheral nervous systems. A healthy gut microbiome is often capable of directly metabolizing (breaking down) neurotoxicants, mitigating potential damage to the host’s nervous system. Should the host’s gut microbiota become unhealthy or abnormal, however, the mitigation of neurotoxicant effects could be compromised, leaving the host’s nervous systems vulnerable to disruption (Dempsey et al., 2019).
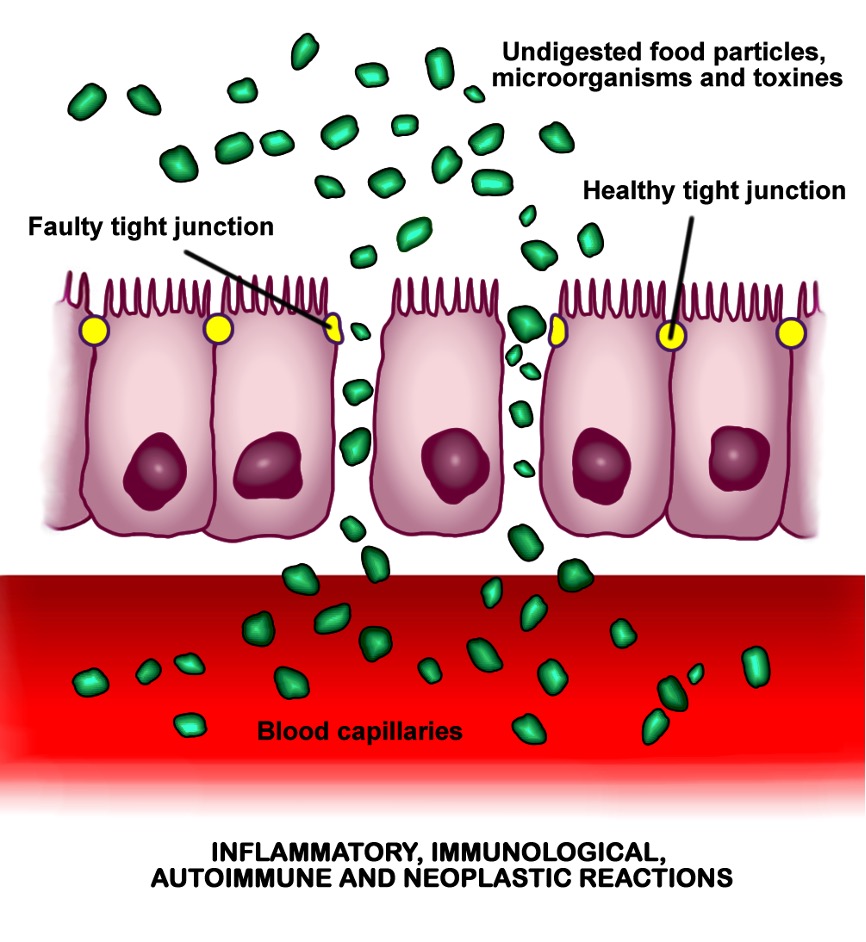
Figure 3: A depiction of “leaky gut” syndrome. Increased gut permeability permits the circulation of bacterial metabolites in the bloodstream.
Source: Wikimedia Commons
Dempsey et al. discuss chemical exposure (such as air pollution, heavy metals, and pesticides) as a stressor that could alter microbiome composition, potentially leading to gut leakiness, as shown in Figure 2 (2019). Such intestinal dysbiosis might involve local inflammation and a subsequent increase in the production of pro-inflammatory cytokines, which, when circulated, could promote the inflammation of nervous tissue. This has been shown to be a risk factor for development of NDs (Castillo et al., 2019).
Dempsey et al. additionally propose that the gut microbiota might influence brain function more directly via the production of microbial metabolites, such as gamma-aminobutyric acid (GABA), which can trigger or inhibit neural responses when circulated to the brain (2019). By inducing neuronal dysfunction, abnormal neurotransmitter and metabolite levels may contribute to the CNS dysfunction present in NDs also.
While much research needs to be done to elucidate precisely how the gut microbiome influences the brain via the microbiota-gut-brain axis and vice-versa, it has become abundantly clear that the brain and gut microbiota’s functions are dependent on each other.
Gut dysbiosis and causes
A key feature of MGBA dysfunction is gut dysbiosis, which as stated above, refers to a change in microbiome composition (Levy et al., 20117). The generality of this definition, however, presents several issues. The identification of dysbiosis by this standard requires an understanding of what characterizes a person’s gut bacterial community as healthy or normal, something that modern researchers are still trying to determine (Shanahan et al., 2021). The basic definition of gut dysbiosis requires a reference population of bacteria to which dysbiotic populations may be compared. However, establishing such a reference population is incredibly challenging due to the diversity of gut microbiomes across the human species, where this diversity results from differences in geography, diet, and age. Differences between individuals’ microbiomes does not automatically mean dysbiosis – some degree of variation between individuals is normal.
Another issue is that while the term “dysbiosis” tends to be associated with disease, an altered bacterial community in the gut is not always harmful to the host, and some changes in microbiome composition can have a neutral or even beneficial effect (Levy et al., 2017). To address these concerns, Levy et al. (2017) favor a narrow definition of gut dysbiosis that emphasizes a state of sustained change in the microbiome that functionally contributes to some disease in the host, rather than a general change in one’s gut microbiome.
Gut dysbiosis can arise from many factors. One potential cause is the proliferation of pathobionts, which are members of the commensal microbiota with pathological capacities. Dysbiosis can also result from a reduction in an individual’s baseline gut bacterial population. This reduction can negatively impact bacterial density, diversity, or both — all of which have been tied to dysbiosis. Additionally, dysbiosis can be caused by certain dietary patterns or the ingestion of xenobiotics (substances like antibiotics that are foreign to the body). For example, mice with high-fat diets have shown reduced microbial diversity. Studies have also found genetics and familial/environmental microbial transmission to be potential causes of gut dysbiosis, along with circadian rhythm disruption, pregnancy, and physical injury (Levy et al., 2017).
It is important to note that the relationship between gut dysbiosis and disease requires further investigation. While some studies have found that altering the gut microbiomes of mice increases their susceptibility to pathogens, the general association of gut dysbiosis with conditions such as autoimmune diseases, allergies, and neurological disorders has yet to be conclusively determined (Wang and Roy, 2017). Some even argue that dysbiosis develops as a result of such conditions rather than contributing to them as a cause (Shanahan et al., 2021). Nonetheless, the growing body of evidence for a role of MGBA dysfunction (often implicating gut dysbiosis) in the etiology of many diseases, including NDs, makes further investigation of the relationship between dysbiosis and disease pathogenesis a research imperative.
Alzheimer’s Disease and the MGBA
Molecular/pathophysiological basis of AD
One ND relevant to MGBA research is Alzheimer’s disease (AD), which affects more than 10% of all Americans older than 65 (Dempsey et al., 2019). AD is also one of the most common causes of dementia worldwide. Current research suggests that an interaction between genetic and environmental factors contributes to AD pathogenesis, with most research focusing on genetic and molecular factors.
One of the most commonly identified AD susceptibility genes is a 3-allele polymorphism (mutation of alleles ε2, ε3, ε4) of apolipoprotein E (ApoE), with the high-risk allele ε4 known to be associated with earlier age of AD onset (Hu et al., 2016). Hallmark pathophysiological traits of AD include extracellular β-amyloid senile plaques (Aβ plaques) and intracellular neurofibrillary tangles (NFT), which are abnormal accumulations of the proteins β-amyloid and tau respectively (Hu et al., 2016; Tiwari et al., 2019). The formation of amyloid plaques starts with the altered cleavage of amyloid precursor protein (APP), an integral protein (transversing) the plasma membrane, to produce insoluble Aβ fibrils (Tiwari et al., 2019). Oligomerized fibrils diffuse into the synaptic clefts of neurons and interfere with synaptic signaling, and over time, they polymerize into aggregations of insoluble fibrils into plaque (Chen and Yan, 2010; Tiwari et al., 2019). In sum, genetic mutations and protein misfolding leads to pathological plaque formation that alters neural function.
MGBA dysfunction as a mechanism of AD etiology
Although much of current AD research focuses on genetic propensities, the rapidly increasing prevalence of AD diverges from Hardy-Weinberg equilibrium (the set of laws describing normal genetic distribution over time), suggesting that environmental factors may play a greater role in AD pathogenesis than previously thought. As a result, scientists have turned to investigate the relationship between environment and AD development (Hu, 2016). As suggested by the gut-microbiota, hygiene, and inflammation hypotheses of AD pathogenesis, one such potential environmentally linked mechanism is dysbiosis induced by ageing and diet, which may lead to AD through systemic inflammation and neuroinflammation (Liang et al., 2018b; Kowalski and Mulak, 2019; Kesika et al., 2020).
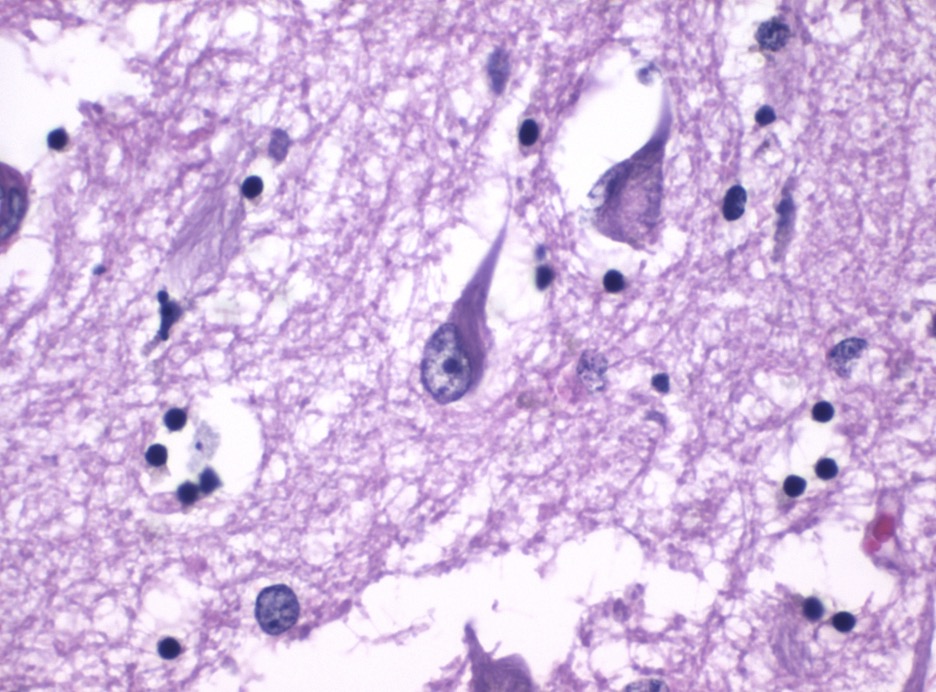
Figure 4: AD neurofibrillary tangles. These structures are a hallmark trait of AD and other tauopathies
Source: Wikimedia Commons
As previously mentioned, Liang et al.’s gut-microbiota hypothesis proposes that NDs may be caused by inflammation-inducing dysbiosis (2018b). The gut-microbiota hypothesis bridges (and is supported by) two additional theories of AD pathogenesis: the hygiene hypothesis and the inflammation hypothesis. According to the hygiene hypothesis, AD is associated with increased environmental sanitation and decreased microbial diversity and is classified as an immunoinflammatory disorder marked by elevated type-1 T helper (Th1) cells, which produce inflammatory signaling molecules IL-2 and TNF-β. (Hu et al., 2016; Romagnani, 1991). Evidence for this theory comes from common patterns of microbiota composition and pathogenesis between patients with certain allergies and patients with AD (Hu et al., 2016).
Similarly, the inflammation hypothesis says that neuroinflammation plays a central role in the development of AD (Kesika et al., 2020). Gut dysbiosis, whether due to lifestyle factors or ageing, is known to cause systemic, or widespread, inflammation (especially in the elderly) via increased gut permeability and circulation of bacterial products (Kowalski and Mulak, 2019; Hu et al., 2016). Because systemic inflammation compromises the blood-brain-barrier, it can lead to neuroinflammation and neurodegeneration (Kowalski and Mulak, 2019). Thus, gut dysbiosis may contribute to AD pathogenesis via immunoinflammatory pathways.
Gut dysbiosis is specifically associated with the accumulation of Aβ plaques, which are recognized as a key immunostimulatory pathophysiological feature of AD (Kesika et al., 2020; Kowalski et al., 2019). Much of current research focuses on the neuroinflammatory effects of these plaques within the context of the brain, but it may be valuable to investigate the role of the gut microbiota in aggravating an immune response against Aβ plaques. Indeed, a similar form of amyloid is abundantly found in the gut as a component of bacterial biofilms; an abundance of amyloid in the gut can lead to mapranosis, or microbial provocation of the neuroinflammatory immune response. Therefore, if gut dysbiosis alters amyloid presence in the gut, it may exacerbate AD-associated inflammation through mapranosis in the brain as well (Kowalski and Mulak, 2019).
Gut dysbiosis can also lead to neuroinflammation and neurodegeneration through altered lipopolysaccharide (LPS) secretion, which is known to induce systemic inflammation and potentially lead to elevated Aβ levels (Kesika et al., 2020; Kowalski and Mulak, 2019). Researchers have demonstrated that elevated levels of LPS in the brain can contribute to many of the features of AD, including excess Aβ (Kowalski and Mulak, 2019). In fact, patients with AD have been shown to exhibit elevated levels of circulating LPS, which is known to induce a pathogenic β-pleating of prion amyloid (Kowalski and Mulak, 2019). It thus seems probable that LPS secretion due to dysbiosis may be involved in the pathogenesis of AD by encouraging Aβ plaque formation.
Parkinson’s Disease and the MGBA
Molecular basis of PD
Parkinson’s disease (PD) is a progressive ND featuring recognizable motor symptoms (such as slow movement, tremors, and postural instability) as well as non-motor symptoms (anosmia/loss of smell, sleep disorders, and gastrointestinal symptoms) (Liddle, 2018; Haehner et al., 2009; Iranzo et al., 2006; Jost, 2010). Non-motor symptoms are shown to sometimes precede the manifestation of motor disturbances by over a decade, suggesting that perhaps nonmotor symptoms, such as GI dysfunction, could indicate something about the root cause of the disorder (Klingelhoefer and Reichmann, 2015).
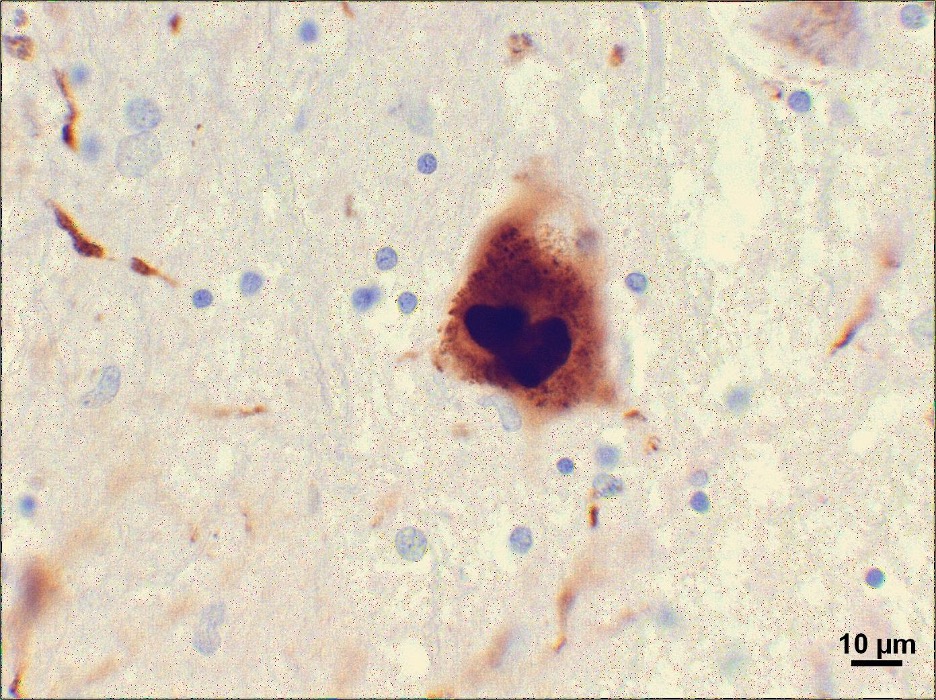
Figure 5: A PD Lewy body. These pathological structures are composed of α-synuclein, a protein associated with the gut which could indicate a MGBA-related etiology of PD
Source: Wikimedia Commons
The key pathophysiological features of PD are the selective degeneration of dopaminergic neurons of the pars compacta (part of the substantia nigra, which is found in the midbrain) and the formation of Lewy bodies, abnormal protein deposits influencing brain activity and behavior (Angot and Brundin, 2009; Bisaglia et al., 2009; Soto, 2012). The loss of dopaminergic neurons leads to the distinctive movement disorder associated with PD and dysfunction of the vagal nerve. A 140 amino-acid protein called α-synuclein is the major protein component of Lewy bodies within the neuron soma, axons, and dendrites and is believed to be involved in the progression of PD (Liddle, 2018). ɑ-synuclein’s tendency to misfold and aggregate allows it to become a template for the misfolding of other ɑ-synuclein molecules, spreading from cell-to-cell like prions (pathogenic misfolded proteins that induce other proteins to misfold as well) (Center for Disease Control and Prevention, 2019; Goedert, 2015). Oligomers, fibrils, and ultimately Lewy bodies form with the accumulation of ɑ-synuclein aggregates (Goedert, 2015; Steiner et al., 2011). It is likely that other regional or cell-autonomous (genetic) factors are also involved in the pathogenesis of PD, such as the fact that patients with PD are known to have particularly long and highly branched axons (Lefers, 2004; Steiner et al., 2011).
MGBA dysfunction as a mechanism of PD etiology
Although PD’s most commonly recognized symptoms are neural, the gastrointestinal symptoms of Parkinson’s point to the MGBA as a potential source of disease etiology. Symptoms including constipation, pain, and nausea often appear in Parkinson’s patients before other neurological signs arise (Liddle, 2018). The appearance of these symptoms both allows for a potential early diagnosis if they are measured, as well as insight into the early stages of the disease.
Additional support for the connection between the gut microbiota and PD is provided by the high expression of α-synuclein in the upper GI tract (Dempsey et al., 2019). The misfolding of α-synuclein has long been recognized as a major contributor to the neural effects of Parkinson’s, and the large concentration of such a key protein for the disease in the enteric nervous system certainly adds to the gut’s relevance in further Parkinson’s studies. In fact, some researchers suggest that the α-synuclein deposits begin in the enteric nervous system up to 20 years in advance of Parkinson’s motor symptoms, and then spread to the central nervous system. This is possible through the “nucleation seed” model, where one misfolded protein can cause surrounding proteins to also misfold, spreading misfolded α-synuclein to the brain where it aggregates as Lewy bodies (Meng et al., 2019).
Further evidence for a gut-to-brain α-synuclein pathogenesis of PD comes from studies in mice that overexpress α-synuclein already. These studies expose the mice to the microbiota of humans with Parkinson’s and use Parkinson’s-free human microbiota as a control. The study found that the microbiota of Parkinson’s patients increased PD-like motor symptoms in the mice, when compared to the control (Dempsey et al., 2019). The influence of the microbiota on the strength of disease symptoms certainly points to a correlative, if not a potentially causative, relationship between the two.
More general support for the connection between Parkinson’s and the gut comes from studies that use chemicals to mimic and induce Parkinson’s symptoms. In one such study, MPTP (1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine, a lipid-soluble toxin) was used to destroy dopaminergic neurons in the basal ganglia of mice, resulting in symptoms strongly resembling those of PD (Sian et al., 1999). In these mice, the MPTP administered affected gut health, even before the Parkinsonian symptoms appeared. In fact, fecal microbiota transplantation from mice exposed to the chemical in the study can cause neural symptoms in non-Parkinson’s mice (Dempsey et al., 2019).
Another drug with similar effects, rotenone, produces Parkinson’s symptoms in rats by destroying both serotonergic and dopaminergic neurons. Its effects are consistent enough that it is a commonly used method of producing mouse models of PD. These mouse models have at least fourteen types of bacteria that are abnormally regulated, either as a direct result of the drug or as a secondary result of neural damage (Dempsey et al., 2019). Further studies are required to determine causation and possible applications.
Future Research Directions
The scientific literature supports the theory that the etiology of certain NDs is not only a result of genetic factors but also a wide variety of environmental and lifestyle factors. As a result, there has been an increased emphasis on potentially changing said environmental and lifestyle factors as a means to prevent and slow ND onset and progress. In line with this effort, there is emerging evidence supporting the role of various MGBA-focused therapies in preventing and mitigating symptoms of NDs.
Nutraceutical and FMT Interventions
For instance, PD pathology has been linked to certain lifestyle habits, and nutraceutical intervention (particularly the use of probiotics, which might someday be individually tailorable through precision medicine) may be a useful complement to traditional therapies. One clinical trial in 2011 showed that fermented milk enriched with Lactobacillus casei improved chronic constipation in individuals with PD while also reducing abdominal pain and swelling. Another trial showed that a probiotic formulation improved insulin resistance and sensitivity, while also improving scores on motor tests for patients with PD (Castelli et. al, 2021).
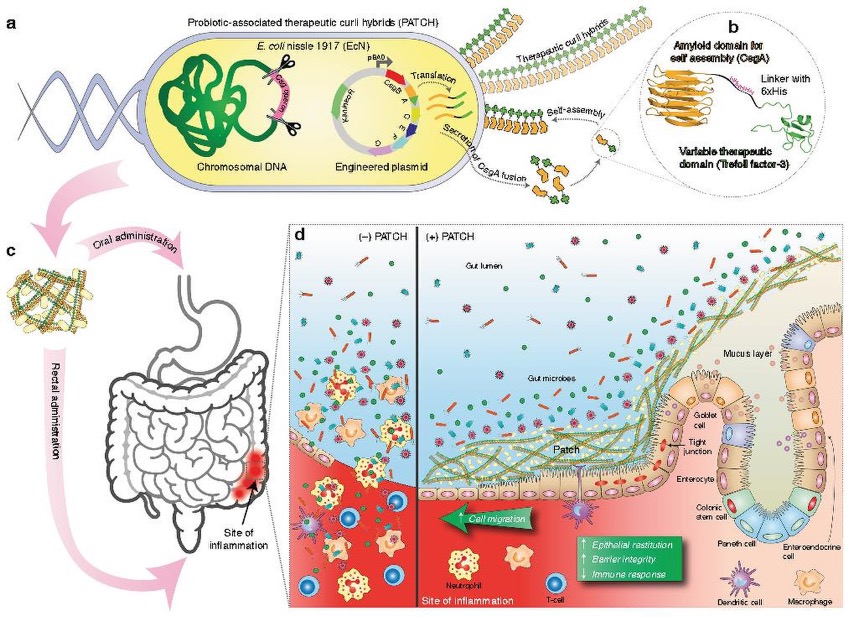
Figure 6: A model for the use of probiotics (probiotic-associated therapeutic curli hybrids, or PATCH) as a strategy for addressing GI-associated immunoinflammatory disorders. (a) E. coli can be engineered to produce therapeutic curli hybrids. (b) Therapeutic curli hybrids consist of an amyloid domain associated with the E. coli biofilm matrix, a linker domain, and a variable therapeutic domain such as a cytokine. (c) The engineered E. coli are administered to a patient either orally or rectally to target an inflamed region of the gut. (d) On the left: without PATCH, release of luminal material and consequent immune activation intensifies inflammation. On the right: with PATCH, the gut-blood barrier is strengthened and immune response is muted, reducing inflammation.
Source: Wikimedia Commons
Probiotics also show promise in mitigating the molecular pathways of PD pathogenesis. Aligned with the finding that α-synuclein misfolding and accumulation begins in the gut, a Caenorhabditis elegans (roundworm) model of synucleinopathy showed that a Bacillus subtilis probiotic inhibited α-synuclein aggregation and cleared pre-formed aggregates. By arresting α-synuclein aggregation from (what is thought to be) the source, the use of probiotics here was shown to both mitigate PD progression and even reverse α-synuclein aggregation in tissues (Castelli et. al, 2021).
Fecal microbiota transplants (FMTs), or the transfer of gut microbiota by introduction of fecal matter from a donor to a recipient, have also shown efficacy as a therapeutic tool for mitigating symptoms of NDs and other neurological disorders. So far, while most studies on FMT for NDs have been conducted on animal subjects, there are examples in the literature of human FMT studies for other neurological conditions. For example, in patients with Autism Spectrum Disorder (ASD), a temporary improvement of ASD and gastrointestinal symptoms was reported after subjects received 8 weeks of oral vancomycin (antibiotic) treatment. A variety of studies focusing on the effects of probiotics in subjects with ASD and in ASD animal models showed improvements in neurobehavioral symptoms such as anxiety and concentration issues as well a reduction in the severity of gastrointestinal symptoms. Additionally, two studies on Multiple Sclerosis (MS), which involves nerve damage due to demyelination of neurons in the CNS, have reported beneficial effects of FMT in slowing MS symptoms and disease progression (Mayo Clinic Staff, 2020; Vendrik et al., 2020). In one instance, FMT was able to resolve a C. difficile infection and also perhaps played a role in preventing MS disease progression for over 10 years (Vendrik et. al, 2020).
While MGBA-focused therapies hold great promise in treating neurodegenerative disorders, research in this field is still ongoing and requires much further work. In one case report, a PD patient who received FMT was observed to have temporary improvement in leg tremors and reduction of PD symptoms; however, only one month later PD symptoms had returned to baseline levels (Vendrik et. al, 2020). Although this case seems to provide evidence that FMT has some beneficial effects, it also shows that these benefits may be temporary. Given that this is simply one example, further research is required to evaluate the impacts of FMT and determine whether FMT therapies can be further developed to improve efficacy.
Need for further study
A handful of issues exist that may hold up the widespread use of MGBA-focused therapies for NDs. The first is the lack of trials with large sample sizes. For example, one case study of a 9-year-old boy with Tourette syndrome found that a FMT procedure performed with a fecal microbiota suspension (collected from a healthy 14-year-old donor) resulted in a dramatic decrease in tic symptoms, which later came back after an initial treatment of tiapride and probiotics (Zhao et al., 2017). However, the authors concluded that future studies should evaluate the long-term effectiveness of FMT and whether any adverse reactions that may occur. Another study of patients with multiple sclerosis (MS) who were administered probiotics found that patients saw a restoration of microbial strains that tend to be absent in MS patients, such as Lactobacillus, along with an anti-inflammatory immune response (Tankou et al., 2018). However, the authors did note that the small sample size may have affected the ability to detect other important differences between the patients and the healthy controls.
Another issue with MGBA-focused therapies lies in the methodological limitations of studies examining them. In a randomized, double-blind, controlled clinical trial of AD patients, compared to control group patients treated with milk alone, patients treated with a mixture of probiotics saw improvements in cognitive abilities, measures of oxidative stress including malondialdehyde (MDA) concentrations, and measures of inflammation including serum high sensitivity C-reactive protein (hs-CRP) concentrations (Akbari et al., 2016). However, the authors also noted difficulty in measuring bacteria loads before and after the AD patients took the probiotic supplements and used only the Mini-Mental State Examination (MMSE) to evaluate cognition (this tests a limited set of cognitive criteria; a more thorough evaluation would be better). In another study of children with autism spectrum disorders (ASD), Kang et al. found that participants showed improvements in gastrointestinal symptoms, behavioral ASD symptoms, and gut microbiota diversity after a microbiota transfer treatment procedure (2017). This suggests that gut microbiome-targeted therapies could be efficacious in treating neurological conditions such as ASD or even NDs. However, because the study was an open-label trial (one which is not placebo controlled, blinded, or randomized), the authors noted that placebo effects (caused by a mere belief that one receives treatment) were likely present.
A final issue with MGBA-focused therapies is a lack of high-power studies examining their effectiveness, where power is a statistical measure of study validity and can be increased, for example, by increasing sample size (Cleary et al., 1970). In a review of an assortment of studies looking at the effects of the gut microbiota on brain function and AD-related pathogenesis, Jiang et al. pointed out a lack of studies investigating the gut microbiota and AD directly (2017). In order to make the use of MGBA-focused therapies more appealing, future studies should aim to address the sample and methodological limitations presented here.
Conclusion
An MGBA-focused approach to preventing and treating NDs shows great promise. Research has suggested that the gut microbiome influences a variety of cognitive and behavioral processes and even contributes to the development of NDs. Therapies such as probiotics, antibiotics, FMT, and dietary interventions can target and manipulate gut microbiota, potentially treating and preventing NDs. The ability of the gut-brain axis to influence a number of bodily systems means that dysbiosis can have profound effects, such as impacting the expression of genes related to different neurotransmitters, impairing immune responses, and contributing to inflammation, all of which appear to be linked to mental disorders and brain diseases (Liang et al., 2018b). Studies have provided evidence that MGBA-focused therapies may be beneficial in combating dysbiosis by increasing or manipulating gut microbiota, supporting their use in treating and preventing NDs.
However, these studies also have significant issues, including small sample sizes and methodological limitations, which prevent their widespread use at present. Although the field of research on the MGBA is growing, there is a lack of high-power studies in the area. Though these limitations may be discouraging, they also reveal a plethora of directions for future research. For instance, researchers may continue to develop novel ways of applying MGBA-focused therapies to patients, such as using combinations of therapies or even using them in tandem with existing treatments. Others may examine the effectiveness of MGBA-focused therapies in patients with different NDs or different populations of patients. Studies can also expand upon the limitations of previously existing studies, perhaps by using larger sample sizes or making use of more rigorous trials.
Despite the challenges that arise with MGBA research, the field has provided researchers with insight into how the gut-brain connection can be applied to treating and preventing NDs. There is no doubt that the field will expand as we continue to elucidate the workings of the MGBA and develop and refine treatments for people with NDs.
References
Akbari, E., Asemi, Z., Daneshvar Kakhaki, R., Bahmani, F., Kouchaki, E., Tamtaji, O. R., Hamidi, G. A., & Salami, M. (2016). Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer’s Disease: A Randomized, Double-Blind and Controlled Trial. Frontiers in Aging Neuroscience, 8. https://doi.org/10.3389/fnagi.2016.00256
Angot, E., & Brundin, P. (2009). Dissecting the potential molecular mechanisms underlying alpha-synuclein cell-to-cell transfer in Parkinson’s disease. Parkinsonism & Related Disorders, 15 Suppl 3, S143-147. https://doi.org/10.1016/S1353-8020(09)70802-8
Bisaglia, M., Mammi, S., & Bubacco, L. (2008). Structural insights on physiological functions and pathological effects of alpha-synuclein. Faseb J, 23, 329–340.
Carding, S., Verbeke, K., Vipond, D. T., Corfe, B. M., & Owen, L. J. (2015). Dysbiosis of the gut microbiota in disease. Microbial Ecology in Health and Disease, 26(s2), 26191. https://doi.org/10.3402/mehd.v26.26191
Castelli, V., d’Angelo, M., Quintiliani, M., Benedetti, E., Grazia Cifone, M., & Cimini, A. (2020, October 9). The emerging role of probiotics in neurodegenerative diseases: New hope for Parkinson’s disease? Castelli V, d’Angelo M, Quintiliani M, Benedetti E, Cifone MG, Cimini A – Neural Regen Res. Neural Regeneration Research. https://www.nrronline.org/article.asp?issn=1673-5374;year=2021;volume=16;issue=4;spage=628;epage=634;aulast=Castelli
Castillo, X., Castro-Obregón, S., Gutiérrez-Becker, B., Gutiérrez-Ospina, G., Karalis, N., Khalil, A. A., Lopez-Noguerola, J. S., Rodríguez, L. L., Martínez-Martínez, E., Perez-Cruz, C., Pérez-Velázquez, J., Piña, A. L., Rubio, K., García, H. P. S., Syeda, T., Vanoye-Carlo, A., Villringer, A., Winek, K., & Zille, M. (2019). Re-thinking the Etiological Framework of Neurodegeneration. Frontiers in Neuroscience, 13. https://doi.org/10.3389/fnins.2019.00728
Center for Disease Control and Prevention. (2019). Prion Diseases | CDC. https://www.cdc.gov/prions/index.html
Chandra, S., Alam, M. T., Dey, J., Sasidharan, B. C. P., Ray, U., Srivastava, A. K., Gandhi, S., & Tripathi, P. P. (2020). Healthy Gut, Healthy Brain: The Gut Microbiome in Neurodegenerative Disorders. Current Topics in Medicinal Chemistry, 20(13), 1142–1153. https://doi.org/10.2174/1568026620666200413091101
Chen, C., Ahn, E. H., Kang, S. S., Liu, X., Alam, A., & Ye, K. (2020). Gut dysbiosis contributes to amyloid pathology, associated with C/EBPβ/AEP signaling activation in Alzheimer’s disease mouse model. Science Advances, 6(31), eaba0466. https://doi.org/10.1126/sciadv.aba0466
Chen, J. X., & Yan, S. S. (2010). Role of mitochondrial amyloid-beta in Alzheimer’s disease. Journal of Alzheimer’s Disease: JAD, 20 Suppl 2, S569-578. https://doi.org/10.3233/JAD-2010-100357
Cleary, T. A., Linn, R. L., & Walster, G. W. (1970). Effect of Reliability and Validity on Power of Statistical Tests. Sociological Methodology, 2, 130–138. https://doi.org/10.2307/270786
Dempsey, J. L., Little, M., & Cui, J. Y. (2019). Gut microbiome: An intermediary to neurotoxicity. NeuroToxicology, 75, 41–69. https://doi.org/10.1016/j.neuro.2019.08.005
Division of Population Health, National Center for Chronic Disease Prevention and Health Promotion. (2020, June 2). What is Alzheimer’s Disease? | CDC. https://www.cdc.gov/aging/aginginfo/alzheimers.htm
Dugger, B. N., & Dickson, D. W. (2017). Pathology of Neurodegenerative Diseases. Cold Spring Harbor Perspectives in Biology, 9(7). https://doi.org/10.1101/cshperspect.a028035
Forsythe, P., Sudo, N., Dinan, T., Taylor, V. H., & Bienenstock, J. (2010). Mood and gut feelings. Brain, Behavior, and Immunity, 24(1), 9–16. https://doi.org/10.1016/j.bbi.2009.05.058
Gao, S., Burney, H. N., Callahan, C. M., Purnell, C. E., & Hendrie, H. C. (2019). Incidence of Dementia and Alzheimer’s Disease Over Time: A Meta-Analysis. Journal of the American Geriatrics Society, 67(7), 1361–1369. https://doi.org/10.1111/jgs.16027
Gerhardt, S., & Mohajeri, M. H. (2018). Changes of Colonic Bacterial Composition in Parkinson’s Disease and Other Neurodegenerative Diseases. Nutrients, 10(6). https://doi.org/10.3390/nu10060708
Goedert, M. (2015). NEURODEGENERATION. Alzheimer’s and Parkinson’s diseases: The prion concept in relation to assembled Aβ, tau, and α-synuclein. Science (New York, N.Y.), 349(6248), 1255555. https://doi.org/10.1126/science.1255555
Haehner, A., Boesveldt, S., Berendse, H. W., Mackay-Sim, A., Fleischmann, J., Silburn, P. A., Johnston, A. N., Mellick, G. D., Herting, B., Reichmann, H., & Hummel, T. (2009). Prevalence of smell loss in Parkinson’s disease—A multicenter study. Parkinsonism & Related Disorders, 15(7), 490–494. https://doi.org/10.1016/j.parkreldis.2008.12.005
Hu, X., Wang, T., Luo, J., Liang, S., Li, W., Wu, X., Jin, F., & Wang, L. (2014). Age-dependent effect of high cholesterol diets on anxiety-like behavior in elevated plus maze test in rats. Behavioral and Brain Functions, 10(1), 30. https://doi.org/10.1186/1744-9081-10-30
Hu, X., Wang, T., & Jin, F. (2016). Alzheimer’s disease and gut microbiota. Science China. Life Sciences, 59(10), 1006–1023. https://doi.org/10.1007/s11427-016-5083-9
Iranzo, A., Molinuevo, J. L., Santamaría, J., Serradell, M., Martí, M. J., Valldeoriola, F., & Tolosa, E. (2006). Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: A descriptive study. The Lancet. Neurology, 5(7), 572–577. https://doi.org/10.1016/S1474-4422(06)70476-8
Jiang, C., Li, G., Huang, P., Liu, Z., & Zhao, B. (2017). The Gut Microbiota and Alzheimer’s Disease. Journal of Alzheimer’s Disease, 58(1), 1–15. https://doi.org/10.3233/JAD-161141
Jost, W. H. (2010). Gastrointestinal dysfunction in Parkinson’s Disease. Journal of the Neurological Sciences, 289(1–2), 69–73. https://doi.org/10.1016/j.jns.2009.08.020
Kang, D.-W., Adams, J. B., Gregory, A. C., Borody, T., Chittick, L., Fasano, A., Khoruts, A., Geis, E., Maldonado, J., McDonough-Means, S., Pollard, E. L., Roux, S., Sadowsky, M. J., Lipson, K. S., Sullivan, M. B., Caporaso, J. G., & Krajmalnik-Brown, R. (2017). Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome, 5(1), 10. https://doi.org/10.1186/s40168-016-0225-7
Kesika, P., Suganthy, N., Sivamaruthi, B. S., & Chaiyasut, C. (2021). Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer’s disease. Life Sciences, 264, 118627. https://doi.org/10.1016/j.lfs.2020.118627
Klingelhoefer, L., & Reichmann, H. (2015). Pathogenesis of Parkinson disease—The gut–brain axis and environmental factors. Nature Reviews Neurology, 11(11), 625–636. https://doi.org/10.1038/nrneurol.2015.197
Kowalski, K., & Mulak, A. (2019). Brain-Gut-Microbiota Axis in Alzheimer’s Disease. Journal of Neurogastroenterology and Motility, 25(1), 48–60. https://doi.org/10.5056/jnm18087
Lefers, Mark. (2004). Cell Autonomous Definition. https://groups.molbiosci.northwestern.edu/holmgren/Glossary/Definitions/Def-C/cell_autonomous.html
Levy, M., Kolodziejczyk, A. A., Thaiss, C. A., & Elinav, E. (2017). Dysbiosis and the immune system. Nature Reviews Immunology, 17(4), 219–232. https://doi.org/10.1038/nri.2017.7
Liang, S., Wu, X., Hu, X., Wang, T., & Jin, F. (2018a). Recognizing Depression from the Microbiota–Gut–Brain Axis. International Journal of Molecular Sciences, 19(6), 1592. https://doi.org/10.3390/ijms19061592
Liang, S., Wu, X., & Jin, F. (2018b). Gut-Brain Psychology: Rethinking Psychology From the Microbiota–Gut–Brain Axis. Frontiers in Integrative Neuroscience, 12. https://doi.org/10.3389/fnint.2018.00033
Liddle, R. A. (2018). Parkinson’s disease from the gut. Brain Research, 1693(Pt B), 201–206. https://doi.org/10.1016/j.brainres.2018.01.010
Luo, J., Wang, T., Liang, S., Hu, X., Li, W., & Jin, F. (2014). Ingestion of Lactobacillus strain reduces anxiety and improves cognitive function in the hyperammonemia rat. Science China Life Sciences, 57(3), 327–335. https://doi.org/10.1007/s11427-014-4615-4
Mayo Clinic Staff. (2020). Multiple sclerosis—Symptoms and causes. Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/multiple-sclerosis/symptoms-causes/syc-20350269
McCann, H., Stevens, C. H., Cartwright, H., & Halliday, G. M. (2014). α-Synucleinopathy phenotypes. Parkinsonism & Related Disorders, 20 Suppl 1, S62-67. https://doi.org/10.1016/S1353-8020(13)70017-8
Meng, L., Yuan, X., Cao, X., & Zhang, Z. (2019). The gut-brain axis in the pathogenesis of Parkinson’s disease. Brain Science Advances, 5(2), 73–81. https://doi.org/10.1177/2096595820902566
National Institute of Environmental Health Sciences. (2019, September 10). Neurodegenerative Diseases. National Institute of Environmental Health Sciences. https://www.niehs.nih.gov/research/supported/health/neurodegenerative/index.cfm
Obrenovich, M. E. M. (2018). Leaky Gut, Leaky Brain? Microorganisms, 6(4). https://doi.org/10.3390/microorganisms6040107
Parkinson’s Foundation. (n.d.). Statistics. Parkinson’s Foundation. Retrieved January 29, 2021, from https://www.parkinson.org/Understanding-Parkinsons/Statistics
Rogers, G. B., Keating, D. J., Young, R. L., Wong, M.-L., Licinio, J., & Wesselingh, S. (2016). From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Molecular Psychiatry, 21(6), 738–748. https://doi.org/10.1038/mp.2016.50
Romagnani, S. (1991). Type 1 T helper and type 2 T helper cells: Functions, regulation and role in protection and disease. International Journal of Clinical & Laboratory Research, 21(2), 152–158. https://doi.org/10.1007/BF02591635
Ronan, V., Yeasin, R., & Claud, E. C. (2021). Childhood Development and the Microbiome—The Intestinal Microbiota in Maintenance of Health and Development of Disease During Childhood Development. Gastroenterology, 160(2), 495–506. https://doi.org/10.1053/j.gastro.2020.08.065
Salas Garcia, M. C., Yee, A. L., Gilbert, J. A., & Dsouza, M. (2018). Dysbiosis in Children Born by Caesarean Section. Annals of Nutrition and Metabolism, 73(3), 24–32. https://doi.org/10.1159/000492168
Sandoval-Motta, S., Aldana, M., Martínez-Romero, E., & Frank, A. (2017). The Human Microbiome and the Missing Heritability Problem. Frontiers in Genetics, 8. https://doi.org/10.3389/fgene.2017.00080
Shanahan, F., Ghosh, T. S., & O’Toole, P. W. (2021). The Healthy Microbiome—What Is the Definition of a Healthy Gut Microbiome? Gastroenterology, 160(2), 483–494. https://doi.org/10.1053/j.gastro.2020.09.057
Sian, J., Youdim, M. B. H., Riederer, P., & Gerlach, M. (1999). MPTP-Induced Parkinsonian Syndrome. Basic Neurochemistry: Molecular, Cellular and Medical Aspects. 6th Edition. https://www.ncbi.nlm.nih.gov/books/NBK27974/
Soto, C. (2012). Transmissible proteins: Expanding the prion heresy. Cell, 149(5), 968–977. https://doi.org/10.1016/j.cell.2012.05.007
Steiner, J. A., Angot, E., & Brundin, P. (2011). A deadly spread: Cellular mechanisms of α-synuclein transfer. Cell Death and Differentiation, 18(9), 1425–1433. https://doi.org/10.1038/cdd.2011.53
Tankou, S. K., Regev, K., Healy, B. C., Tjon, E., Laghi, L., Cox, L. M., Kivisäkk, P., Pierre, I. V., Hrishikesh, L., Gandhi, R., Cook, S., Glanz, B., Stankiewicz, J., & Weiner, H. L. (2018). A probiotic modulates the microbiome and immunity in multiple sclerosis. Annals of Neurology, 83(6), 1147–1161. https://doi.org/10.1002/ana.25244
Tiwari, S., Atluri, V., Kaushik, A., Yndart, A., & Nair, M. (2019, July 19). Alzheimer’s disease: Pathogenesis, diagnostics, and therapeutics. International Journal of Nanomedicine; Dove Press. https://doi.org/10.2147/IJN.S200490
Vendrik, K. E. W., Ooijevaar, R. E., de Jong, P. R. C., Laman, J. D., van Oosten, B. W., van Hilten, J. J., Ducarmon, Q. R., Keller, J. J., Kuijper, E. J., & Contarino, M. F. (2020). Fecal Microbiota Transplantation in Neurological Disorders. Frontiers in Cellular and Infection Microbiology, 10. https://doi.org/10.3389/fcimb.2020.00098
Wang, F., & Roy, S. (2017). Gut Homeostasis, Microbial Dysbiosis, and Opioids. Toxicologic Pathology, 45(1), 150–156. https://doi.org/10.1177/0192623316679898
Wang, T., Hu, X., Liang, S., Li, W., Wu, X., Wang, L., & Jin, F. (2015). Lactobacillus fermentum NS9 restores the antibiotic induced physiological and psychological abnormalities in rats. Beneficial Microbes, 6(5), 707–717. https://doi.org/10.3920/BM2014.0177
Weiss, G. A., & Hennet, T. (2017). Mechanisms and consequences of intestinal dysbiosis. Cellular and Molecular Life Sciences, 74(16), 2959–2977. https://doi.org/10.1007/s00018-017-2509-x
Zhao, H., Shi, Y., Luo, X., Peng, L., Yang, Y., & Zou, L. (2017, December 12). The Effect of Fecal Microbiota Transplantation on a Child with Tourette Syndrome [Case Report]. Case Reports in Medicine; Hindawi. https://doi.org/10.1155/2017/6165239
Related Posts
Overview, History, Essential Concepts, and Current Topics in Transplant Immunology
First Author: Anahita Kodali1 Co-Authors: Vaishavi Agrawal2, Vivek Babu3, Sakeena...
Read MoreHow the Human Microbiome Disables Drugs (And What We Can About It)
Scientists are studying the human microbiome and its ability to...
Read MoreA Spoonful of (Modified) Sugar as an Antiviral Medicine
Figure 1: A negative stain transmission electron micrograph of Influenza...
Read More