Source: HHMI BioInteractive
Treatment of chronic respiratory infections, especially in individuals with cystic fibrosis (CF), has been challenging due to bacterial resistance and polymicrobial interactions. However, a recent study has introduced a breakthrough diagnostic approach named AtbFinder, which aims to transform antibiotic selection by considering the complexities of polymicrobial communities in the CF lung1.
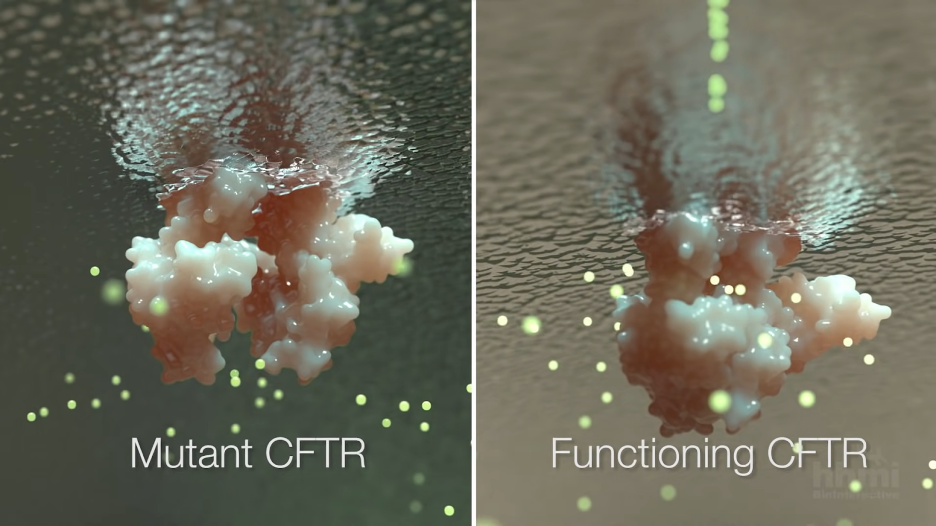
The cystic fibrosis transmembrane conductance regulator (CFTR) is ineffective in CF. It is responsible for transporting chlorine and other ions into the airways, helping to keep them hydrated2. When CFTR is not working properly, the airways become backed up with thick, sticky mucus that impairs breathing and creates a haven for pathogenic microbes like the bacterial pathogen P. aeruginosa1. P. aeruginosa is very common in people with CF. In fact, P. aeruginosa infection is associated with worsening CF symptoms3.
Traditionally, antibiotics are prescribed to CF patients based on antimicrobial susceptibility tests (ASTs), which isolate a single pathogen from patient sputum samples (a coughed-up mixture of saliva and mucus), grow them in the lab, and expose them to a panel of different antibiotics to see which is most effective. However, ASTs often fail for CF patients – antibiotics that are effective in the lab are ineffective against the bacteria in the CF lungs1.
A major factor behind the failure of traditional antibiotic susceptibility testing (AST) techniques is the existence of polymicrobial infections: multiple pathogens living in the CF lung at once. The lead pathogen-centric methodology ignores the complex dynamics of the microbial ecosystems in the lungs. The fact that P. aeruginosa persists in CF patients even after intensive antibiotic therapy highlights the shortcomings of the current AST techniques1.
AtbFinder is unlike conventional AST techniques in that it uses a unique TGV nutritional medium, which enables the cultivation of a more diversified selection of bacteria from polymicrobial communities in the CF lung. The system evaluates the effectiveness of antibiotics against mixed biofilms containing multiple different bacteria to stimulate the complex interbacterial interactions that are taking place in the lungs. Another benefit of AtbFinder is that it chooses antibiotics according to the concentrations reached in the lungs (according to prior scientific literature), giving a more realistic picture of the host environment1.
To test the effectiveness of antibiotic selection with AtbFinder, a study enrolled 35 individuals with CF, with 33 of them having chronic P. aeruginosa colonization. The patients had clinical data from the several years prior to using AtbFinder and were followed for several years after. AtbFinder was consistently able to prescribe effective antibiotics, leading to an 81.8% clearance of P. aeruginosa in subsequent cultures after treatment with AtbFinder-prescribed antibiotics. In addition, the number of pulmonary exacerbations (acute worsening of lung function that often leads to hospitalization and IV antibiotic treatment) decreased and forced expiratory volume in 1 second (FEV1%), a common measurement of lung function in people with CF, increased by 28.4% from baseline on average1. There was also a dramatic reduction in the number of antibiotic courses needed after using AtbFinder1.
The efficacy of AtbFinder in enhancing microbiological and clinical results points to a potential paradigm shift in the use of antibiotics for complex illnesses. The study results have wider ramifications for managing polymicrobial illnesses besides cystic fibrosis, such as chronic obstructive pulmonary disease (COPD). AtbFinder’s clinical value is further enhanced by its faster turnaround time (4 hours) compared to traditional AST techniques (48 hours)1. AtbFinder stands out as a promising tool with the potential to transform antibiotic therapy. Some of the researchers who developed the system have started a company called TVG-DX (lead author George Tetz is the CEO and last author Victor Tetz is the CSO). They are currently conducting additional clinical trials that could bring their system within reach for many more patients.
References
- Tetz, G., Kardava, K., Vecherkovskaya, M., Hahn, A., Tsifansky, M., Koumbourlis,
A., & Tetz, V. (2023). AtbFinder diagnostic test system improves optimal
selection of antibiotic therapy in persons with cystic fibrosis. Journal of
Clinical Microbiology, 61(1). https://doi.org/10.1128/jcm.01558-22 - Basics of the CFTR Protein. (n.d.). Cystic Fibrosis Foundation. Retrieved
December 10, 2023, from https://www.cff.org/research-clinical-trials/
basics-cftr-protein#:~:text= - Rosenfeld, M., Faino, A. V., Onchiri, F., Aksit, M. A., Blackman, S. M., Blue,
E. E., Collaco, J. M., Gordon, W. W., Pace, R. G., Raraigh, K. S., Zhou,
Y.-H., Cutting, G. R., Knowles, M. R., Bamshad, M. J., & Gibson, R. L.
(2022). Comparing encounter-based and annualized chronic pseudomonas
infection definitions in cystic fibrosis. Journal of Cystic Fibrosis,
21(1), 40-44. https://doi.org/10.1016/j.jcf.2021.07.020 - CFTR. (n.d.). John Hopkins Cystic Fibrosis Center. Retrieved December 10, 2023,
from https://hopkinscf.org/knowledge/cftr/ - Bernatowicz, R., & Peereboom, D. (2016). Lessons learned. Glioblastoma, 279-290.
https://doi.org/10.1016/b978-0-323-47660-7.00024-0
Related Posts
Incarceration and Motherhood: A Brief Overview of Maternal Health Issues in American Prisons
Figure 1: Guards in prisons are able to put prisoners...
Read MoreUnderstanding the Social Factors Affecting Cancer Therapy
Cover Image: A patient being prepared for radiation therapy. (Source:...
Read MoreCOVID-19 and its Implications for Adolescent Mental Health
Covid 19 and Isolation (Source: created by author) Abstract The...
Read MoreType 2 Diabetes in Hopewell, Virginia: A Public Health Perspective
This publication is in proud partnership with Project UNITY’s Catalyst Academy 2024...
Read MoreTransgender Health Disparities in Prisons
Transgender people are targets for victimization within the prison system...
Read MoreMedical Overtreatment in the United States: Causes, Consequences, and Solutions
This article was originally submitted to the Modern MD competition...
Read MoreNoel Chazhur