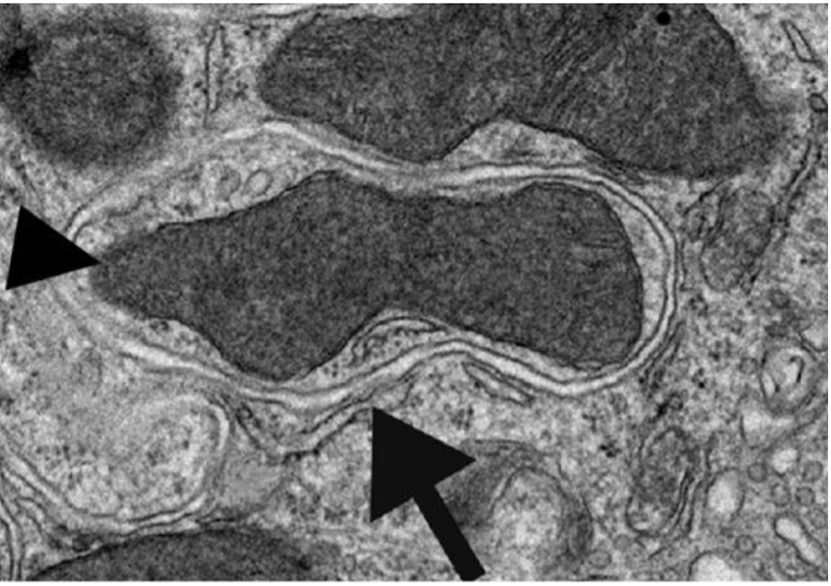
Figure 1: The bottom arrow points to an autophagic vesicle engulfing and beginning to degrade the mitochondria (indicated by the arrowhead on the left) in addition to other cytoplasmic material
Source: Wikimedia Commons
Autophagy is an important process by which cells degrade and recycle damaged or unnecessary components (Rees, 2020). Currently, autophagy is a key research area because better understanding of its mechanism could have implications for the development of therapies for cancer, which is often associated with autophagic defects. One characteristic that makes autophagy particularly interesting, as well as complicated, is its dual nature with respect to cancer treatment. In the early stages of cancer, promotion of autophagy could act as a tumor suppressor by causing the self-destruction of cancer cells. However, promotion of autophagy in later stages of tumor progression could enhance the ability of cancer cells to survive and metastasize by allowing them to cope with harsh environmental conditions. This is the case because autophagy degrades and discards dangerous cellular components, thus making it easier for affected cancer cells to survive and proliferate (Bhutia et al., 2015). Clearly, a better working knowledge of the details concerning autophagy is necessary for effective therapeutic progress.
One recent discovery involves endonuclease G (ENDOG), a mitochondrial enzyme whose most prevalent role is the degradation of nuclear DNA during apoptosis, or programmed cell death (van Loo et al., 2001). Scientists at the University of Jinan in China looked into ENDOG’s role in multiple pathways that impact levels of autophagic activity.
They found that one way ENDOG promotes autophagy is via suppression of the mTOR signaling pathway, a “well-known negative regulator of autophagy” (Wang et al., 2020). Essentially, the mTOR signaling pathway, central to the regulation of many key cellular functions, is highly associated with cell growth, survival, and proliferation (Laplante & Sabitini, 2009). It follows logically that there is downregulation of the mTOR pathway while a cell undergoes autophagy; it is not sensible for a cell to proliferate without having taken care of detected damaged components that could potentially be dangerous to cell stability.
Findings regarding the mechanism of this suppression revealed that ENDOG first binds to a protein (14-3-3γ) involved in the regulation of mTOR signaling. This binding results in the release of two other proteins, TSC2 and Vps34, that are negative regulators of mTOR. The mTOR pathway is inhibited as a result, thereby permitting the initiation of autophagy. Critically, the phosphorylation of two ENDOG amino acids, T128 and S288, is required for its interaction with 14-3-3γ and ability to promote autophagy (Wang et al., 2020).
This pathway, although significant, is not the only mechanism implicated as part of ENDOG’s role in autophagy promotion. Researchers also found that ENDOG was able to help induce autophagy by activating and/or enhancing the DNA damage response. ENDOG plays an important role in the DNA damage response through its endonuclease activity. Among other enzymes, it is able to cleave damaged DNA sequences into fragments, helping to push the cell toward the autophagic response. When the DNA in question becomes too damaged to be repaired, signaling events occur that lead to autophagy or apoptosis (Eliopoulos et al., 2016).
These findings taken together are significant in that they provide a better understanding of autophagy and specifically the role ENDOG plays in its regulation. ENDOG itself is potentially a crucial player to consider in developing strategies for cancer treatment, as research shows that breast cancer cells expressing higher levels of ENDOG are more often found to undergo activation of programmed cell death (Basnakian et al., 2006). Continuing and expanding research to understand the role of ENDOG and other mechanisms in autophagy will have significant implications for the field of medicine.
References
Basnakian, Alexei G, et al. (2006). “Endonuclease G Promotes Cell Death of Non-Invasive Human Breast Cancer Cells.” Experimental Cell Research, U.S. National Library of Medicine, www.ncbi.nlm.nih.gov/pmc/articles/PMC1839947/.
Bhutia, Sujit K, et al. (2015). “Autophagy: Cancer’s Friend or Foe?” Advances in Cancer
Research, U.S. National Library of Medicine, www.ncbi.nlm.nih.gov/pubmed/23768510.
Eliopoulos, Aristides et al. (2016). “DNA Damage Response and Autophagy: A Meaningful Partnership.” Frontiers in Genetics, Frontiers Media S.A., www.ncbi.nlm.nih.gov/pmc/articles/PMC5116470/.
Laplante, Mathieu, and David M. Sabatini. (2009). “MTOR Signaling at a Glance.” Journal of Cell Science, The Company of Biologists Ltd, jcs.biologists.org/content/122/20/3589#:~:text=The%20mammalian%20target%20of%20rapamycin,%2C%20growth%2C%20proliferation%20and%20survival.&text=These%20observations%20have%20attracted%20broad%20scientific%20and%20clinical%20interest%20in%20mTOR.
Rees, Mathieu. (2020). “Autophagy: Definition, Health Effects, Fasting, and More.” Medical News Today, MediLexicon International, www.medicalnewstoday.com/articles/autophagy.
van Loo, G, et al. (2001). “Endonuclease G: a Mitochondrial Protein Released in Apoptosis and Involved in Caspase-Independent DNA Degradation.” Cell Death and Differentiation, U.S. National Library of Medicine, www.ncbi.nlm.nih.gov/pubmed/11753562.
Wang, Wenjun, et al. (2020). “Endonuclease G Promotes Autophagy by Suppressing MTOR Signaling and Activating the DNA Damage Response.” Nature News, Nature Publishing Group, www.nature.com/articles/s41467-020-20780-2.
Related Posts
Your Personality Might Say Less About Your Brain, And More About Your Microbiome
Figure 1: A scanning electron micrograph of Enterococcus faecalis, a bacterium...
Read MoreThe Mediterranean Diet Could Help Treat and Prevent Adolescent Depression
Source: Mediterranean Diet Pyramid (Flikr Public Domain, Zaid Alasad) The...
Read MoreA Spoonful of (Modified) Sugar as an Antiviral Medicine
Figure 1: A negative stain transmission electron micrograph of Influenza...
Read MoreMatthew Lutchko