First Author: Jillian Troth1
Co-Authors [Alphabetical Order]: Roxanna Attar2, Caroline Conway1, Julia Gainski3, Allison Kifer4, Vincent Lai5, Grace Lee6, Callie Moody1, Melanie Prakash1, Julia Schwab7
1Dartmouth, 2University of Texas at Austin, 3University of Illinois at Urbana-Champaign, 4University of California San Diego, 5Rice University, 6Williams College, 7Danube Private University, Austria

Figure 1: Depression is a serious mood disorder affecting millions of people which often manifests in symptoms of anhedonia (the inability to feel pleasure), helplessness, and suicidal thoughts
Source: Pixabay.com, Małgorzata Tomczak
Introduction
Studying Depression
Depression is a mental illness affecting over 350 million people worldwide (Limbana et al., 2020). One of the most common mood disorders, depression detrimentally affects a patient’s quality of life by interfering with social life, school/work, and even basic self-care. If left untreated, depression can lead to an increased risk of morbidity and mortality (Silva et al., 2020). Therefore, understanding and effectively treating this illness is an imperative goal on which overall societal well-being depends.
Causes of depression include one’s environment and their internal biology. Environmentally, depression may be caused by factors such as grief over a death or loss, apathy, particular medications, substance misuse, and a wide array of other life factors translating into a loss in self-esteem. Biologically, patients who experience clinical depression might have certain genetic mutations, disruption of neurogenesis (neuron formation), and/or neurotransmitter deficiencies (Delgado, 2000).
Recent research also indicates that depression may be linked to dysfunction of the microbiota-gut-brain axis (MGBA). A healthy gut microflora transmits signals to the brain through endocrine, immunological, and neurological pathways – all of which will be discussed throughout the course of this paper. Dysfunction in any of these pathways tends to be detrimental to health. Notably, individuals with depression tend to exhibit a dysregulation of neuroendocrine and neuroimmune pathways, as well as Inflammatory Bowel Disease (IBD) (Limbana et al., 2020).
Given the close connection between the gut and the brain, studying the gut microbiome may prove essential to understanding the specific causes of depression and the severity and length of depressive symptoms (Flux & Lowry, 2019). Thus, this paper will investigate the value in studying Major Depressive Disorder (MDD) from the perspective of the MGBA.
The MGBA
The MGBA refers to a bidirectional communication between the brain and gastrointestinal tract, involving the central nervous system (CNS), the autonomic nervous system (ANS), the enteric nervous system (ENS), and the hypothalamic pituitary adrenal (HPA) axis (Carabotti et al., 2020). Composed of the brain and spinal cord, the CNS is the main “processing center” for the entire nervous system (Thau et al., 2020). The ANS, which regulates unconscious bodily functions such as digestion, heart rate, and metabolism, drives both afferent (sensory) and efferent (muscular) signals in the nervous system (Schmidt & Thews, 1989; Hall et al., 1990).
One of the largest divisions of the autonomic nervous system is the ENS, involving over 30 neurotransmitters and other circulating hormones and peptides that cross the blood-brain barrier (Bonaz et al., 2017). Through these signaling pathways, the ENS acts synergistically with the vagus nerve. A major contributor to the parasympathetic nervous system, the vagus nerve regulates a variety of crucial bodily functions such as mood control and digestion (Breit et al., 2018).
Stress response and other bodily functions are also regulated via the hypothalamus-pituitary-adrenal (HPA) axis, a neuroendocrine system composed of the hypothalamus, the pituitary gland, and the adrenal gland (Liu & Zhu, 2018). Evidence suggests that the gut microbiome interacts with both the HPA axis and vagus nerve through bacterial metabolites (products), thereby affecting a variety of physiological processes (Kaur et al., 2019). Additionally, as intestinal microorganisms play a role in maintaining the balance of the host’s immune system, the gut-brain axis impacts immune function (Kim & Shin, 2018).
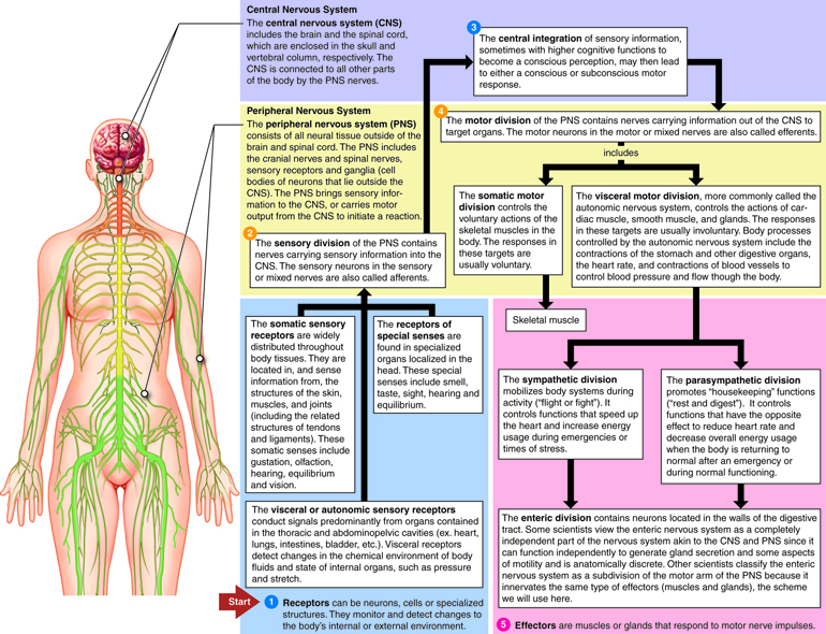
Figure 2: The nervous system consists of many branches, including the CNS, ANS, ENS, and HPA axis. The CNS connects all parts of the body, integrating information coming in from the sensory division of the PNS, and relaying information out to the motor division of the PNS. Under the umbrella of the motor division, the ENS is the nervous system of the digestive tract. The MBGA connects the ENS to the CNS via the vagus nerve and impacts the HPA axis via bacterial metabolites
Source: Neurotech.edu, Cenveo
The gut microbiome consists of all the microbes in an individual’s intestines and may even be viewed as another organ that contributes to one’s general health. An individual’s gut microbiome is unique, as it depends on factors such as diet, age, metabolism, medication use and history, stress, growth and development, genetics, and environment. The microbiome performs many essential functions – it helps in the digestion of fiber, breast milk, and control of the immune system, and it also works to promote general brain health (Robertson, 2017).
Recent studies on germ-free rodents (those lacking a microbiome), antibiotics, and gastrointestinal tract infections also emphasize the important role the gut microbiome plays in regulating the nervous system through the MGBA (Cryan & Dinan, 2015; Kim & Shin, 2018). Given the accumulating evidence in support of the gut microbiota’s central role in various essential processes in the body, the gut-brain axis is important to consider when studying and developing treatments for depression and other mood-related neurological disorders (Stilling et al., 2014).
This article will summarize findings from these studies, looking at four different pathways of the MGBA — endocrine, metabolic, immune, and neurological — and the role that they play in the development of depression.
Endocrine Pathways of the MGBA and Depression
The endocrine system is a network of hormone-producing glands that constitutes the main chemical messaging system for the body. The hormones released by the endocrine system are vital for almost every organ in the body, and play a role in puberty, stress, and metabolism (Stephens et al., 2012). One integral part of the endocrine system is the HPA axis. This axis has three major parts, as indicated by the name. The first section is the hypothalamus, a part of the forebrain. It controls the second section – the pituitary glands. The pituitary has two distinct parts: the anterior pituitary gland is controlled by a hormonal signal that travels from the hypothalamus, while the posterior pituitary is controlled directly by neurons that extend from the hypothalamus. These glands hormonally control the third section of the HPA axis, the adrenal gland, which consists of the adrenal medulla and cortex. The adrenal medulla triggers a short-term, intense stress response known as the adrenaline response. The adrenal cortex, the other part of the adrenal gland, handles the response to long-term, chronic stress. As a whole, the HPA axis acts as a pathway to trigger and maintain the body’s response to stressors (Stephens et al., 2012).
Because of its involvement with the body’s stress response, the HPA axis is closely tied with depression. All manners of stress trigger the HPA axis and therefore the release of stress hormones throughout the body. Such hormones include vasopressin from the posterior pituitary and cortisol from the adrenal cortex, and they can have widespread effects relating to an abnormal stress response (Stephens et al., 2012). The cascade of stress hormones is supposed to operate as a negative feedback circuit, meaning that increased hormone levels in the bloodstream should decrease their production, keeping these stress hormones at reasonable levels. However, a majority of people with depression exhibit dysfunctional negative feedback, causing these hormone levels to rise and become imbalanced (Foster & Neufield, 2013).
The activity of the HPA axis has been tied to the MGBA in a variety of ways. For one, stress has been shown to increase the permeability of the gut, allowing gut microbes and their molecular products to escape into the bloodstream. This gut ‘leakiness’ can cause inflammation and induce other aspects of the immune response (Du et al., 2020). The immune response can activate the release of small proteins called cytokines, some of which activate the HPA axis. Additionally, some of the microbiota in the gut can even cross the blood-brain barrier and affect HPA axis response in the brain directly (Misiak et al., 2020). Furthermore, the gut microbiota can have an organizational effect on the HPA axis. In other words, through a variety of shared biochemical interactions, the microbiota present early in life may impact the long-term development of the HPA axis.
Because of this link, abnormalities in the MGBA can influence depressive symptoms, and treatments targeting the MGBA can potentially be used to influence depression. Some studies have found that a variety of probiotics can positively affect depression symptoms, and that treated patients can display lower levels of stress hormones (Du et al., 2020). Studies in mice have found that transferring gut microbes from subjects with depression to those without it can induce anxiety and depression-like symptoms in the latter. Subsequent treatment with probiotics reduced these symptoms and had a direct impact on the HPA axis by reducing levels of hippocampal RNA transcripts related to HPA axis regulation (Misiak et al., 2020). In rats, probiotics administered during postnatal development were found to stabilize the HPA axis long-term after development (Gareau et al., 2007). Overall, the HPA-axis is a highly probable contributing factor for depression due to the close ties between depression and chronic stress. Furthermore, connections between the HPA axis and the MGBA may also explain the impact of treatments like probiotics on depressed patients.
Metabolic Pathways of the MGBA and Depression
SCFAs
Metabolically, the MGBA may influence depressive symptoms through short chain fatty acid (SCFA) production. By absorbing and fermenting starches resistant to digestion as well as complex carbohydrates such as dietary fiber, the gut microbiota produce these fatty acid molecules (Liu et al., 2018; Silva et al., 2020). SCFA production is heavily affected by factors such as fiber intake as well as the amount and species type of microbes present (Silva et al., 2020). Bacterial SCFA production is extremely important in maintaining homeostasis, as colonic SCFA levels affect gut health and are thought to influence the activity of the MGBA (Silva et al., 2020; Liu et al., 2018).
In particular, the SCFAs butyrate, acetate, and propionate have neuroactive properties, as they can enter the blood and access the brain through the G-protein coupled receptors FFA2 and FFA3 (Liu et al., 2018). Growing evidence suggests that these SCFAs may influence brain physiology and impact mood and behavior, although the underlying mechanisms are not well understood (Delgado, 2000). For example, the evidence for the neuroactivity of SCFAs supports the hypothesis that dysbiosis affects mood disorders. It was shown, first in naturally depressed rodents and later in non-human primates, that SCFA levels are decreased in depressed subjects compared to non-depressed subjects (Feng-Li, 2019). Additionally, fecal SCFA concentrations were found to be lower in depressed human patients (Silva et al., 2020).
This correlation between depression, dysbiosis, and lower SCFA levels might be explained in part by the SCFA butyrate’s innate antidepressant-like effects. In murine models, depressive symptoms like low energy, anhedonia (the inability to feel pleasure), and sociability impairments were reversed with chronic administration of butyrate (Wei, 2015). Furthermore, depressive and manic-like behaviors in rats were reversed with the administration of sodium butyrate (Resende, 2013). Finally, the effects of psychosocial stress in rats were mitigated with SCFA treatment, as demonstrated by open field test observations (specifically, the finding that treated rats spent more time in the central region of an open field, indicating lack of stress) and decreased expression of stress-signaling receptor genes (van de Wouw, 2018). Evidently, the constitution of one’s gut microbiome affects the level of SCFAs present in the body, which in turn, have been shown through murine models to have widespread effects on depressive and stress-related disorders.
LPS
As discussed in previous sections, the gut microbiome includes a plethora of bacteria that are able to metabolize or provide essential nutrients to the body. However, some bacterial metabolites or components can be harmful when in overabundance. Lipopolysaccharides (LPS) are one example – they are a structural component produced by and attached to the surface membrane of gram-negative bacteria (Wassenaar & Zimmermann, 2018).
The structure of LPS consists of a long chain of sugar molecules covalently attached to lipids. Embedded in the bacterial cell membrane, LPS acts as a first line of defense, protecting the bacteria from toxic molecules such as bile salts and lipophilic antibiotics (Bertani & Ruiz, 2018). LPS also functionally stabilizes the cell membrane. Over time, in order to keep the integrity of its membrane, the bacteria constantly replenish LPS, releasing older molecules into the surrounding space (Liu & Zhu, 2018). Lipid A, one lipid component of LPS molecules, is responsible for helping bacteria ward off chemical attacks. But when secreted, it can have harmful effects on the human host (Wassenaar & Zimmermann, 2018).
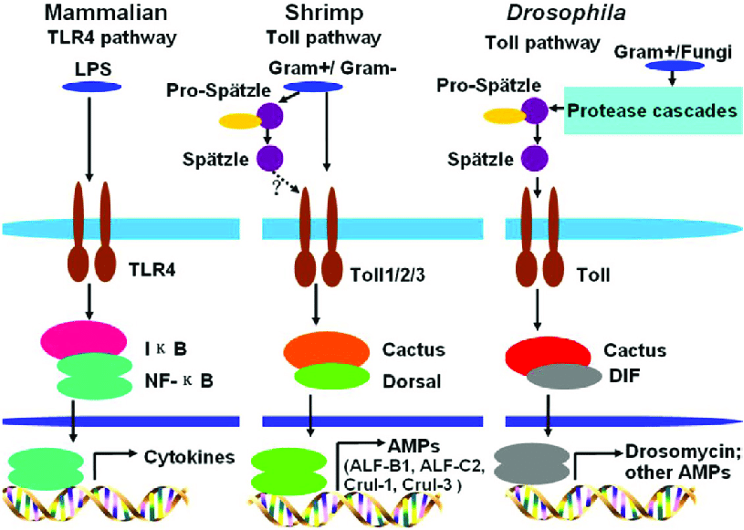
Figure 3: Schematic depiction of the effects of bacterial metabolites on host gene expression. In mammals, bacterial LPS induces cytokine production via the TLR4 pathway, resulting in an inflammatory immune response
Source: Wikimedia Commons, Sun et al., 2017
LPS is a very potent and chemically active molecule. One critical molecule that LPS activates is indoleamine 2,3 dioxygenase (IDO), an enzyme involved in inflammation (O’Connor et al., 2009). Depression rates have been found to be elevated in patients with chronic inflammatory conditions such as atherosclerosis and rheumatoid arthritis as well as patients that are taking cytokine immunotherapy treatments which also heighten the inflammatory response (O’Connor et al., 2009). Therefore, one might reason that elevated IDO may prompt depressive symptoms by stimulating inflammation. In fact, an increase in IDO activation has been correlated with increases in clinical depression rates and depressive symptoms (Liu & Zhu, 2018).
In one study, LPS was injected into mice and consequently led to an increase in depressive behaviors, observed as decreased mobility in a swim test as well as tail suspension test (a measurement of physical activity by taping a strain gauge to the tail), decreased food consumption, and decreased sexual activity (O’Connor et al., 2009). Others have also postulated that LPS is responsible for sickness behaviors, a specific subset of symptoms within major depressive disorder patients. These behaviors can include low energy, psychomotor slowness, irritability, and anorexia (van Eeden et al., 2020). In a study conducted by Dr. van Eeden and colleagues at the Leiden University Medical Center, LPS-stimulated inflammatory markers were more prevalent among patients with sickness behaviors of depression than those with non-sickness behaviors of depression.
Immunological Pathways of the MGBA and Depression
The human immune system protects against microbes and other harmful substances that enter the body; due to the immune system’s close involvement with the microbiome, it is an essential factor to consider when studying the MGBA and associated conditions (Chaplin, 2010). In fact, the gut itself comprises a major component of the immune system; the gut-associated lymphoid tissue (GALT) serves to protect the intestinal mucosa from invasion (Lee & Mazmanian, 2010). Thus, as the gut is important to the immune system, the gut microbiota may play a role in its regulation.
Recent research connecting inflammation and depression hints at a link between MGBA dysfunction and major depressive disorder (MDD). One study has compared the GALT and gut microbiota of Dark Agouti (DA) rats, which are susceptible to induced experimental autoimmune encephalomyelitis (EAE), and the microbiota of Albino Oxford (AO) rats, which were resistant to EAE. The researchers found several differences in the immune cells and gut microbiota across the two groups of rats. EAE rats, it should be noted, are used as a model organism for the study of the human disease multiple sclerosis (Stanisavljević et al., 2016).
Specifically, the researchers found that within the GALT, DA rats featured a higher proportion of CD4+ T cells and regulatory T cells and produced more of the pro-inflammatory cytokines interferon-γ and interleukin-17 than the AO rats. This elevation of inflammation-associated molecules and cells in DA rats suggests a link between the inflammatory immune response and EAE. In addition, the researchers found that AO rats, but not DA rats, hosted species of the bacterial genus Turicibacter and genus Atopostipes in their feces and intestines, respectively. This difference in microbiota constitution between the groups of rats distinguished by inflammatory tendencies supports the hypothesis that gut dysbiosis is associated with differences in inflammatory immune reactivity (Stanisavljević et al., 2016).
Historically, germ-free animal models have also provided valuable insight into the role played by the gut microbiota in immune regulation. For example, in a study where researchers exposed different groups of guinea pigs to the bacteria Shigella flexneri, Sprinz and colleagues found that exposure to S. flexneri proved fatal for germ-free guinea pigs (1961). However, when exposed first to a strain of Escherichia coli, the bowels of the germ-free guinea pigs more closely resembled those of the conventionally raised guinea pigs, and they were protected from fatal S. flexneri infection (Sprinz et al., 1961).
While these studies provide evidence for the role of the gut microbiota in rat and guinea pig immune function, future research should consider how the gut microbiota interacts with the human immune system. According to one review, while the link between dysbiosis and human disease is uncertain, it should be considered as a potential contributor to autoimmune and inflammatory diseases such as multiple sclerosis (MS) and rheumatoid arthritis (RA) as dysbiosis may offset the balance between inflammatory and anti-inflammatory bacteria (Lee & Mazmanian, 2010).
Notably, there is also considerable evidence for the role of the immune system in depression, perhaps indicating a link between the MGBA and MDD. Specifically, the inflammatory hypothesis of depression posits that inflammation may contribute to depression symptoms. One way that it does this is through the action of proinflammatory cytokines, which travel throughout the body to signal an immune response (Gałecki & Talarowska, 2018). As previously discussed in relation to LPS-induced inflammation, this inflammatory immune response often manifests in “sickness behavior” including lethargy, decreased appetite, depressed mood, heightened sensitivity to pain, and difficulty concentrating (Flux & Lowry, 2019).
Figure 4: Artistic rendering of neuroinflammation on the cellular level. Neuroinflammation has been linked to both MGBA dysfunction and depression, indicating that the MGBA may influence neurological function
Source: Flickr, National Institute on Aging, NIH
In a meta-analysis of twenty-four studies measuring cytokine concentration in patients with major depression, Dowlati and colleagues found that there was an increased concentration of proinflammatory cytokines interleukin factor (IL)-6 and tumor necrosis factor (TNF)-α in patients with major depression compared to control subjects (2010). In other words, inflammatory molecules were associated with depression, suggesting a link between inflammation and depression. Further, Müller and Schwarz propose a mechanism by which cytokines might induce depressive symptoms, focusing on the enzyme indoleamine 2,3-dioxygenase (IDO), which is activated by proinflammatory cytokines (2007). IDO breaks down tryptophan, which is required for serotonin synthesis, and its cytokine-induced activation may be associated with serotonin deficit, which is also implicated in depression (Grohmann et al., 2003). Put simply, proinflammatory molecules such as cytokines are thought to induce depression by activating the enzyme IDO, whose function ultimately decreases serotonin availability, where serotonin is a neurotransmitter associated with depression when in deficit.
Finally, the surface membrane of the gut itself is involved in an immunoinflammatory mechanism of depression. In a review discussing the involvement of the gut in MDD, Rudzki and Maes describe how MDD is associated with increased intestinal permeability, or “leaky gut,” which can allow bacterial LPS to induce inflammation by activating the production of proinflammatory cytokines (2020). They also discuss how certain beneficial gut microbes can lower proinflammatory cytokine levels. These may be useful to consider when developing novel treatments for depression.
Neurological Pathways of the MGBA and Depression
Vagal Signaling and Neurotransmitters
The MGBA is tied to the vagus nerve, which facilitates bidirectional neural signaling between the intestines and the brain, but primarily conducts signals toward the central nervous system. The gut microbiota is capable of activating the vagus nerve and regulating intestine-brain communication (Kim & Shin, 2018).
In addition to activation of the vagus nerve, gut microbes can influence nervous system function through the production of neurotransmitters. The neurotransmitters produced can include GABA (gamma-aminobutyric acid, the main inhibitory neurotransmitter in the central nervous system), dopamine (the “feel-good” neurotransmitter), and serotonin (a neurotransmitter which, among other things, regulates mood) (Kim & Shin, 2018). Researchers estimate that more than 90% of the body’s serotonin and 50% of its dopamine are synthesized in the gut (Liang et al., 2018a). These microbiota-produced neurotransmitters can induce neural signaling by binding to nerve receptors in the ENS. In addition to producing the neurotransmitters themselves, gut microbiota may impact the expression of neurotransmitter-related genes. Scientists have observed that such genes are expressed differently in populations of mice raised in totally germ-free conditions versus mice lacking one specific microbe (Liang et al., 2018a).
Dysfunction of the neurotransmitter GABA is associated with depression. In one study on mice, specific bacterial strains were shown to reduce depressive behaviors while increasing hippocampal concentrations of GABA (Kim & Shin, 2018). Importantly, the gut bacterial strains were only effective at regulating depressive behavior with an intact vagus nerve. Two specific gut bacterial strains in humans (Lactobacillus brevis and Bifidobacterium dentium) are known to produce GABA in significant quantities, and have therefore been hypothesized to regulate depressive behavior in people in a way comparable to that observed in mice (Kim & Shin, 2018).
Another study found that feeding the bacterium L. rhamnosus to male mice not only led to a reduction in depressive behaviors but also altered the expression of GABA receptors in parts of the mice’s brains. This effect, too, was only observed when the vagus nerve was intact (Foster & McVey Neufeld, 2013). A different study found that probiotic supplementation in rats decreased depressive symptoms — again, however, cutting the vagus nerve made such treatment ineffective (Liang et al., 2018b). In sum, the results of these GABA studies indicate that the vagus nerve is essential to the gut microbiota’s regulation of central nervous system activity. They also show that changes to the gut microbiota can contribute to (or mitigate) depressive behavior.
In addition to GABA, other neurotransmitters have been implicated. The monoaminergic neurotransmitter deficiency hypothesis asserts that insufficient amounts of serotonin, norepinephrine, and/or dopamine result in depressive symptoms too (Liang et al., 2018a). Knowing that more than 90% of the body’s serotonin and 50% of its dopamine are synthesized in the gut, it is reasonable to suggest that the MGBA could be tied to depression and its associated deficiencies in serotonin/dopamine levels (Liang et al., 2018b). More research is necessary, however, to elucidate the specifics of this role and to determine if MGBA dysfunction leads to depression or vice-versa.
BDNF
Another potential neurological way in which the MGBA influences depression symptoms involves brain derived neurotrophic factor (BDNF), a protein expressed in areas of the brain that ultimately develop neurons involved in learning and memory. BDNF is expressed highly in neuronal areas involved in excitatory and inhibitory signaling, as well as areas that have activity-dependent plasticity. Its transcription is dependent upon activation by different promoters, suggesting that different environmental or biological triggers can stimulate expression of genes that lead to certain BDNF levels (Miranda et al., 2019). BDNF has been shown to be involved in regulating glucose and energy metabolism by binding to the tyrosine kinase receptor, and BDNF is thought to induce expression of transcription factors to encourage neuronal stem cell differentiation (Ito et al., 2003; Bathina & Das, 2015). Importantly, low levels of BDNF often correlate with several neurodegenerative diseases like Parkinson’s or Alzheimer’s (Bathina & Das, 2015).
Research has demonstrated the role of BDNF in the MGBA and neurological function. In one study, germ free mice displayed discrepancies in memory function, which the study traces back to low levels of BDNF. Another study found that different microbiome compositions (cultivated by application of antimicrobial medications that targeted specific species) could lead to differential BDNF expression in the brain (Carabotti et al., 2015). Yet another study demonstrated that psychobiotic treatments (prebiotics and/or postbiotics used to improve mental health via the MGBA) can ameliorate depression; the effects of such treatments were measured by correlations between BDNF and depression symptoms (Heidarzadeh-Rad et al., 2020).
According to the neurotrophic hypothesis of depression, decreased neuroplasticity may be an effect of lower BDNF levels (Dwivedi, 2009). Accordingly, low levels of BDNF and a resulting lack of neuroplasticity have also been found to be associated with depression and suicidal thoughts. It is therefore not a surprise that antidepressants are thought to upregulate BDNF to increase neural plasticity (Dwivedi, 2009). However, despite abundant evidence for the neurotrophic hypothesis of depression, some studies argue that BDNF may be less a cause of depression and more of a symptom which can be used as a metric of treatment efficacy (Lee & Kim, 2010; Kishi et al., 2017). Regardless of the precise cause-and-effect relationship, it is generally agreed that low BDNF activity is a marker of depression, and more specifically, suicidality.
Conclusion: Prospective Therapeutics and Future Directions
Clinical Trials
MGBA dysfunction is becoming a new focus of depression treatment. There have been four major therapies suggested to normalize the gut microbiota and MGBA function: oral intake of probiotics, prebiotics, a healthy diet, and fecal microbiota transplantation (FMT) (Liang et al., 2018a). Whereas prebiotics and healthy diets only nourish beneficial bacteria that are already in the gut, FMT and probiotics colonize the gut with new healthy bacteria. Consequently, most research on these four therapies focuses on the psychobiotics (probiotics and prebiotics), given that their intake is more appealing than FMT (Liang et al., 2018a). However, two main issues pervading probiotic therapy research are the diversity and severity of depressive symptoms (as both are often very subjective), as well as variation between probiotic supplements. Different probiotics have been found to have significantly different effects (Yong et al., 2020).
Systematic reviews have shown that lactic acid bacteria (such as Lactobacillus) and bifidobacteria, as probiotic species, bring forth similar effects as antidepressants in patients with major depressive disorder (MDD) (Yong et al., 2020). One study directly compared four subject groups given either Lactobacillus probiotics, citalopram (an antidepressant), both, or neither to determine the effects of probiotics for stress-induced depression (Abdrabou et al., 2018). Mice treated with solely probiotics showed a positive change in depression biomarkers (like kynurenine) and symptoms compared with those untreated. However, there was no significant difference in the success of using only probiotics versus only antidepressants. Ultimately, combining Lactobacilli probiotics and citalopram was the most successful therapy for stress-induced depression in mice; such a combined therapy should be investigated further (Abdrabou et al., 2018).
Another study examined the combination of probiotics, magnesium orotate, and selective serotonin reuptake inhibitors, or SSRIs (another class of antidepressants), for the mitigation of treatment-resistant depression (Bambling et al., 2017). The researchers found that depressive symptoms were alleviated for eight weeks, the duration of probiotic treatment, but then returned when patients were switched to only SSRIs (Bambling et al., 2017).
Figure 5: Phase contrast microscopic image of Lactobacillus acidophilus, a bacteria found in the gut and commonly used in probiotics
Source: Wikimedia Commons, Josef Reischig
Taking this into account, probiotics should be considered as a possible depression treatment, and like antidepressants, may be individualized depending on the depressive disorder and the patient’s unique microbiome composition. For now, though, probiotics should only be used as an adjuvant therapy to other antidepressants until there is further evidence in favor of their use.
Next Steps
Major depressive disorders affect at least 6.7% of the adult U.S. population; furthermore, with the current pandemic, this prevalence is estimated to have risen three-fold (Anxiety & Depression Association of America, 2021). Treatments including antidepressants often don’t fully improve symptoms, and have side effects like loss of libido, suicidality, emotional numbness, and addiction (Yong et al, 2020). Furthermore, patients taking antidepressants often feel stigmatized as emotionally weak, which does not contribute to their healing. Therefore, treatments with higher response rates and fewer side effects should be investigated. Lately, treatment of MGBA dysfunction as a part of depression pathogenesis has shown potential as a therapeutic alternative; probiotic therapy, for example, is not associated with a social stigma and appears efficacious in reducing depressive symptoms (Yong et al., 2020).
While an MGBA-focused approach to depression treatment has only recently been investigated, a few researchers have inquired into the side effects of probiotics. For example, while evidence supports the general safety of probiotics, in some cases immunocompromised patients or those suffering from leaky-gut-syndrome could be endangered as probiotics could cause sepsis, pneumonia, allergies and endocarditis (infection-induced cardiac inflammation) (Didari et al., 2014; Yong et al., 2020; Mayo Clinic Staff, 2020). Considering these life-threatening risks, probiotics should be treated as any other medication and therefore tested properly before marketing. Furthermore, pharmacists should advise patients and be aware of potential side effects.
Within the next few years, MGBA-focused depression therapies such as healthy diet, FMT, and particularly psychobiotics seem likely to become important components of a refocused treatment of depression. Notably, the antidepressant paradigm of depression treatment may be challenged and modified by such MGBA-informed therapies in the near future, hopefully providing more efficacious options for those suffering from depression.
References
Abdrabou, A. M., Osman, E. Y., & Aboubakr, O. A. (2018). Comparative therapeutic efficacy study of Lactobacilli probiotics and citalopram in treatment of acute stress-induced depression in lab murine models. Human Microbiome Journal, 10, 33–36. https://doi.org/10.1016/j.humic.2018.08.001
ADAA. (2021). Facts & Statistics | Anxiety and Depression Association of America, ADAA. https://adaa.org/understanding-anxiety/facts-statistics
Bambling, M., Edwards, S. C., Hall, S., & Vitetta, L. (2017). A combination of probiotics and magnesium orotate attenuate depression in a small SSRI resistant cohort: An intestinal anti-inflammatory response is suggested. Inflammopharmacology, 25(2), 271–274. https://doi.org/10.1007/s10787-017-0311-x
Bathina, S., & Das, U. N. (2015). Brain-derived neurotrophic factor and its clinical implications. Archives of Medical Science, 11(6), 1164–1178. https://doi.org/10.5114/aoms.2015.56342
Bertani, B., & Ruiz, N. (2018). Function and biogenesis of lipopolysaccharides. EcoSal Plus, 8(1). https://doi.org/10.1128/ecosalplus.ESP-0001-2018
Bonaz, B., Sinniger, V., & Pellissier, S. (2017). Vagus nerve stimulation: A new promising therapeutic tool in inflammatory bowel disease. Journal of Internal Medicine, 282(1), 46–63. https://doi.org/10.1111/joim.12611
Breit, S., Kupferberg, A., Rogler, G., & Hasler, G. (2018). Vagus Nerve as Modulator of the Brain–Gut Axis in Psychiatric and Inflammatory Disorders. Frontiers in Psychiatry, 9. https://doi.org/10.3389/fpsyt.2018.00044
Carabotti, M., Scirocco, A., Maselli, M. A., & Severi, C. (2015). The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Annals of Gastroenterology, 28(2), 203–209.
Chaplin, David D. (2010). Overview of the immune response—Journal of Allergy and Clinical Immunology. https://www.jacionline.org/article/S0091-6749(09)02837-1/fulltext
Cryan, J. F., & Dinan, T. G. (2015). Gut microbiota: Microbiota and neuroimmune signalling-Metchnikoff to microglia. Nature Reviews. Gastroenterology & Hepatology, 12(9), 494–496. https://doi.org/10.1038/nrgastro.2015.127
Delgado, P. L. (2000). Depression: The case for a monoamine deficiency. The Journal of Clinical Psychiatry, 61 Suppl 6, 7–11.
Deng, F.-L., Pan, J.-X., Zheng, P., Xia, J.-J., Yin, B.-M., Liang, W.-W., Li, Y.-F., Wu, J., Xu, F., Wu, Q.-Y., Qu, C.-H., Li, W., Wang, H.-Y., & Xie, P. (2019). Metabonomics reveals peripheral and central short-chain fatty acid and amino acid dysfunction in a naturally occurring depressive model of macaques. Neuropsychiatric Disease and Treatment, 15, 1077–1088. https://doi.org/10.2147/NDT.S186071
Didari, T., Solki, S., Mozaffari, S., Nikfar, S., & Abdollahi, M. (2014). A systematic review of the safety of probiotics. Expert Opinion on Drug Safety, 13(2), 227–239. https://doi.org/10.1517/14740338.2014.872627
Dowlati, Yekta, Herrmann, Nathan, Swardfager, Walter, Liu, Helena, Sham, Lauren, Reim, Elyse K., & Lanctot, Krista L. (2009). A Meta-Analysis of Cytokines in Major Depression—Biological Psychiatry. https://www.biologicalpsychiatryjournal.com/article/S0006-3223(09)01229-3/fulltext
Du, Y., Gao, X.-R., Peng, L., & Ge, J.-F. (2020). Crosstalk between the microbiota-gut-brain axis and depression. Heliyon, 6(6). https://doi.org/10.1016/j.heliyon.2020.e04097
Dwivedi, Y. (2009). Brain-derived neurotrophic factor: Role in depression and suicide. Neuropsychiatric Disease and Treatment, 5, 433–449.
Flux, M. C., & Lowry, C. A. (2020). Finding intestinal fortitude: Integrating the microbiome into a holistic view of depression mechanisms, treatment, and resilience. Neurobiology of Disease, 135, 104578. https://doi.org/10.1016/j.nbd.2019.104578
Foster, J. A., & Neufeld, K.-A. M. (2013). Gut–brain axis: How the microbiome influences anxiety and depression. Trends in Neurosciences, 36(5), 305–312. https://doi.org/10.1016/j.tins.2013.01.005
Gałecki, P., & Talarowska, M. (2018). Inflammatory theory of depression. Psychiatria Polska, 52(3), 437–447. https://doi.org/10.12740/PP/76863
Gareau, M. G., Jury, J., MacQueen, G., Sherman, P. M., & Perdue, M. H. (2007). Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut, 56(11), 1522–1528. https://doi.org/10.1136/gut.2006.117176
Grohmann, Ursula, Fallarino, Francesca, & Puccetti, Paolo. (2003). Tolerance, DCs and tryptophan: Much ado about IDO: Trends in Immunology. https://www.cell.com/trends/immunology/fulltext/S1471-4906(03)00072-3?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS1471490603000723%3Fshowall%3Dtrue
Hall, W. D. (1990). An Overview of the Autonomic Nervous System. In H. K. Walker, W. D. Hall, & J. W. Hurst (Eds.), Clinical Methods: The History, Physical, and Laboratory Examinations (3rd ed.). Butterworths. http://www.ncbi.nlm.nih.gov/books/NBK399/
Heidarzadeh-Rad, N., Gökmen-Özel, H., Kazemi, A., Almasi, N., & Djafarian, K. (2020). Effects of a Psychobiotic Supplement on Serum Brain-derived Neurotrophic Factor Levels in Depressive Patients: A Post Hoc Analysis of a Randomized Clinical Trial. Journal of Neurogastroenterology and Motility, 26(4), 486–495. https://doi.org/10.5056/jnm20079
Ito, H., Nakajima, A., Nomoto, H., & Furukawa, S. (2003). Neurotrophins facilitate neuronal differentiation of cultured neural stem cells via induction of mRNA expression of basic helix-loop-helix transcription factors Mash1 and Math1. Journal of neuroscience research, 71(5), 648–658. https://doi.org/10.1002/jnr.10532
Kaur, H., Bose, C., & Mande, S. S. (2019). Tryptophan Metabolism by Gut Microbiome and Gut-Brain-Axis: An in silico Analysis. Frontiers in Neuroscience, 13. https://doi.org/10.3389/fnins.2019.01365
Kim, Y.-K., & Shin, C. (2018). The Microbiota-Gut-Brain Axis in Neuropsychiatric Disorders: Patho-physiological Mechanisms and Novel Treatments. Current Neuropharmacology, 16(5), 559–573. https://doi.org/10.2174/1570159X15666170915141036
Kishi, T., Yoshimura, R., Ikuta, T., & Iwata, N. (2018). Brain-Derived Neurotrophic Factor and Major Depressive Disorder: Evidence from Meta-Analyses. Frontiers in Psychiatry, 8. https://doi.org/10.3389/fpsyt.2017.00308
Lee, B.-H., & Kim, Y.-K. (2010). The Roles of BDNF in the Pathophysiology of Major Depression and in Antidepressant Treatment. Psychiatry Investigation, 7(4), 231–235. https://doi.org/10.4306/pi.2010.7.4.231
Lee, Y. K., & Mazmanian, S. K. (2010). Has the Microbiota Played a Critical Role in the Evolution of the Adaptive Immune System? Science, 330(6012), 1768–1773. https://doi.org/10.1126/science.1195568
Liang, S., Wu, X., Hu, X., Wang, T., & Jin, F. (2018a). Recognizing Depression from the Microbiota–Gut–Brain Axis. International Journal of Molecular Sciences, 19(6), 1592. https://doi.org/10.3390/ijms19061592
Liang, S., Wu, X., & Jin, F. (2018b). Gut-Brain Psychology: Rethinking Psychology From the Microbiota–Gut–Brain Axis. Frontiers in Integrative Neuroscience. http://dx.doi.org.dartmouth.idm.oclc.org/10.3389/fnint.2018.00033
Limbana, T., Khan, F., & Eskander, N. (2020). Gut Microbiome and Depression: How Microbes Affect the Way We Think. Cureus, 12(8). https://doi.org/10.7759/cureus.9966
Liu, L., & Zhu, G. (2018). Gut–Brain Axis and Mood Disorder. Frontiers in Psychiatry, 9. https://doi.org/10.3389/fpsyt.2018.00223
Mayo Clinic Staff. (2020, November 14). Endocarditis—Symptoms and causes. Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/endocarditis/symptoms-causes/syc-20352576
Miranda, M., Morici, J. F., Zanoni, M. B., & Bekinschtein, P. (2019). Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Frontiers in Cellular Neuroscience, 13. https://doi.org/10.3389/fncel.2019.00363
Misiak, B., Łoniewski, I., Marlicz, W., Frydecka, D., Szulc, A., Rudzki, L., & Samochowiec, J. (2020). The HPA axis dysregulation in severe mental illness: Can we shift the blame to gut microbiota? Progress in Neuro-Psychopharmacology and Biological Psychiatry, 102, 109951. https://doi.org/10.1016/j.pnpbp.2020.109951
Müller, N., & Schwarz, M. J. (2007). The immune-mediated alteration of serotonin and glutamate: Towards an integrated view of depression. Molecular Psychiatry, 12(11), 988–1000. https://doi.org/10.1038/sj.mp.4002006
Munzone, E., & Colleoni, M. (2015). Clinical overview of metronomic chemotherapy in breast cancer. Nature Reviews Clinical Oncology, 12(11), 631–644. https://doi.org/10.1038/nrclinonc.2015.131
O’Connor, J. C., Lawson, M. A., André, C., Moreau, M., Lestage, J., Castanon, N., Kelley, K. W., & Dantzer, R. (2009). Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Molecular Psychiatry, 14(5), 511–522. https://doi.org/10.1038/sj.mp.4002148
Resende, W. R., Valvassori, S. S., Réus, G. Z., Varela, R. B., Arent, C. O., Ribeiro, K. F., Bavaresco, D. V., Andersen, M. L., Zugno, A. I., & Quevedo, J. (2013). Effects of sodium butyrate in animal models of mania and depression: Implications as a new mood stabilizer. Behavioural Pharmacology, 24(7), 569–579. https://doi.org/10.1097/FBP.0b013e32836546fc
Robertson, Ruairi. (2017, June 27). Why the Gut Microbiome Is Crucial for Your Health. Healthline. https://www.healthline.com/nutrition/gut-microbiome-and-health
Rudzki, L., & Maes, M. (2020). The Microbiota-Gut-Immune-Glia (MGIG) Axis in Major Depression. Molecular Neurobiology, 57(10), 4269–4295. https://doi.org/10.1007/s12035-020-01961-y
Schmidt, A; Thews, G (1989). “Autonomic Nervous System”. In Janig, W (ed.). Human Physiology (2 ed.). New York, NY: Springer-Verlag. pp. 333–370.
Silva, Y. P., Bernardi, A., & Frozza, R. L. (2020). The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Frontiers in Endocrinology, 11. https://doi.org/10.3389/fendo.2020.00025
Sprinz, H., Kundel, D. W., Dammin, G. J., Horowitz, R. E., Schneider, H., & Formal, S. B. (1961). The Response of the Germ-free Guinea Pig to Oral Bacterial Challenge with Escherichia coli and Shigella flexneri. The American Journal of Pathology, 39(6), 681–695.
Stanisavljević, S., Lukić, J., Momčilović, M., Miljković, M., Jevtić, B., Kojić, M., Golić, N., Mostarica Stojković, M., & Miljković, D. (2016). Gut-associated lymphoid tissue, gut microbes and susceptibility to experimental autoimmune encephalomyelitis. Beneficial Microbes, 7(3), 363–373. https://doi.org/10.3920/BM2015.0159
Stephens, M. A. C., & Wand, G. (2012). Stress and the HPA Axis. Alcohol Research : Current Reviews, 34(4), 468–483.
Stilling, R. M., Dinan, T. G., & Cryan, J. F. (2014). Microbial genes, brain & behaviour—Epigenetic regulation of the gut-brain axis. Genes, Brain, and Behavior, 13(1), 69–86. https://doi.org/10.1111/gbb.12109
Thau, L., Reddy, V., & Singh, P. (2021). Anatomy, Central Nervous System. In StatPearls. StatPearls Publishing. http://www.ncbi.nlm.nih.gov/books/NBK542179/
van Eeden, W. A., van Hemert, A. M., Carlier, I. V. E., Penninx, B. W. J. H., Lamers, F., Fried, E. I., Schoevers, R., & Giltay, E. J. (2020). Basal and LPS-stimulated inflammatory markers and the course of individual symptoms of depression. Translational Psychiatry, 10(1), 1–12. https://doi.org/10.1038/s41398-020-00920-4
Wassenaar, T. M., & Zimmermann, K. (2018). Lipopolysaccharides in Food, Food Supplements, and Probiotics: Should We be Worried? European Journal of Microbiology & Immunology, 8(3), 63–69. https://doi.org/10.1556/1886.2018.00017
Wei, Y. B., Melas, P. A., Wegener, G., Mathé, A. A., & Lavebratt, C. (2015). Antidepressant-Like Effect of Sodium Butyrate is Associated with an Increase in TET1 and in 5-Hydroxymethylation Levels in the Bdnf Gene. International Journal of Neuropsychopharmacology, 18(pyu032). https://doi.org/10.1093/ijnp/pyu032
Wouw, M. van de, Boehme, M., Lyte, J. M., Wiley, N., Strain, C., O’Sullivan, O., Clarke, G., Stanton, C., Dinan, T. G., & Cryan, J. F. (2018). Short-chain fatty acids: Microbial metabolites that alleviate stress-induced brain–gut axis alterations. The Journal of Physiology, 596(20), 4923–4944. https://doi.org/10.1113/JP276431
Yang, T., Nie, Z., Shu, H., Kuang, Y., Chen, X., Cheng, J., Yu, S., & Liu, H. (2020). The Role of BDNF on Neural Plasticity in Depression. Frontiers in Cellular Neuroscience, 14. https://doi.org/10.3389/fncel.2020.00082
Yong, S. J., Tong, T., Chew, J., & Lim, W. L. (2020). Antidepressive Mechanisms of Probiotics and Their Therapeutic Potential. Frontiers in Neuroscience, 13. https://doi.org/10.3389/fnins.2019.01361
Yong-Ku, K., & Cheolmin, S. (2018). The Microbiota-Gut-Brain Axis in Neuropsychiatric Disorders: Pathophysiological Mechanisms and Novel Treatments. Current Neuropharmacology, 16(5), 559–573.
Related Posts
Bad dreams? According to new studies, COVID-19 could be to blame
Figure 1: A sleeping child. When we sleep, we dream,...
Read MoreThe Effects of Affirmative Action on Healthcare Diversity
This publication is in proud partnership with Project UNITY’s Catalyst...
Read MoreGut Bacteria May be a Cause of Colorectal Cancer (and the Key to Effective Treatment)
Figure: Cancer cell division is rapid and uncontrollable. New evidence...
Read MoreJillian Troth, Roxanna Attar, Caroline Conway, Julia Gainski, Allison Kifer, Vincent Lai, Grace Lee, Callie Moody, Melanie Prakash, Julia Schwab