Authors: Sakeena Badrane1, Vivek Babu2, Sanah Handu1
1University of Pittsburgh, 2Drexel University
Source: Wikimedia Commons
Introduction
Since the onset of the pandemic, the American populace has lived in varying levels of isolation. In March, the country entered a complete lockdown. Fear of the unknown in conjunction with warnings from the CDC resulted in most Americans completely isolating for months. In June, some of this fear dissipated and amenities gradually reopened. But still, social distancing guidelines were enforced. In November, cases reached unprecedented levels and still continue to rise. As a result, many households are returning to the lockdown state observed in the early pandemic.
Throughout these turbulent times, several lifestyle changes became commonplace. Contact with others was limited in accordance with social distancing guidelines. Exceptional hygienic practices were utilized by many families, such as carrying hand sanitizer and limiting contact with surfaces. The closure of many workplaces, summer camps, and schools continued, forcing many to explore new pastimes and adapt to online school and work formats. This ultimately resulted in the resurgence of parks and other outdoor spaces, serving as a safe space to escape the confines of the home. These drastic lifestyle changes will undoubtedly have some lasting effect on the American population, especially for highly impressionable children.
Our paper will focus on the possible relationship between the pandemic and the “Old Friends” Hypothesis, the reformed Hygiene Hypothesis. The “Old Friends” Hypothesis states that humans must be exposed to symbiotic microbes during childhood in order for adaptive immunity to properly develop. Children primarily encounter these bacteria through contact with others and the outdoors. Interestingly, the pandemic has greatly affected both of these opportunities to acquire beneficial microbes. This connection draws attention to the potential effects on children’s adaptive immunity, and whether these effects are beneficial or harmful. Additionally, the under-exposure to “old friends” may increase susceptibility to severe COVID-19 symptoms. These are possibilities we aim to address in our review.
History and Evolution of the Hygiene Hypothesis
One of the first observations about how infectious pathogens contribute to immune dysregulation was B.M. Greenwood’s finding in 1969 that Western Nigeria had a low incidence of rheumatoid arthritis. He theorized that frequent exposure to malaria might contribute to this disparity (Stiemsma et al., 2015). A year later, Greenwood further supported this theory by observing suppressed spontaneous autoimmune disease in mice infected with Plasmodium berghei, which causes rodent malaria (Stiemsma et al., 2015). In 1976, Gerrard et al. found this same disparity between rural and urbanized communities by finding a decreased rate of allergies in indigenous populations in Northern Canada compared to urban Caucasian populations (Stiemsma et al., 2015).
The origins of the classic Hygiene Hypothesis can be traced to David P. Strachan’s 1989 paper, “Hay fever, hygiene, and household size” in the British Medical Journal. Strachan substantiated this theory in 1996 by examining family history, medical records, and allergy skin prick test results in 11,765 children and finding that household size was inversely correlated with the development of hay fever (Stiemsma et al., 2015). He hypothesized that this trend arose because children can be exposed to germs by older siblings, developing a degree of immunity to hay fever. In contrast, a lack of early childhood exposure to unhygienic conditions can increase an individual’s susceptibility to allergies (Strachan, 1989).
Strachan’s hypothesis was later expanded upon by Dr. Erika von Mutius in the late 1990s. Mutius’ study centered on comparing the rates of allergies and asthma in children who grew up in former East and West Germany. Mutius’ initial hypothesis was that East German children, who grew up in less hygienic and poorer environmental conditions (heavy air pollution, for example), would exhibit incidences of allergies and asthma greater than their Western counterparts (Mutius, 2006). However, her research found the opposite: children in the polluted areas of East Germany had lower allergic reactions and fewer cases of asthma than children in West Germany. Mutius’ work suggested that children who are around numerous other children or animals early in life are exposed to more microbes, and their immune systems develop more tolerance for the antigens that cause asthma (Mutius, 2010).
However, Strachan’s observations about the epidemiological disparities in allergic diseases did not have an immunological backing, until Dr. Tim Mosmann and Dr. Robert Coffman’s findings about the T-helper 1 (Th1) and T-helper 2 (Th2) cell subtypes. Mosmann and Coffman found that T-cells in mice secreted two separate cytokine profiles: the proinflammatory Th1 state and the anti-inflammatory Th2 state (Stiemsma et al., 2015). Th2 cells play a primary role in the allergen sensitization process. Infection with viruses and intracellular bacteria stimulates Th1 immune responses, which suppress Th2 cytokine activity through proinflammatory cytokines (Stiemsma et al., 2015).
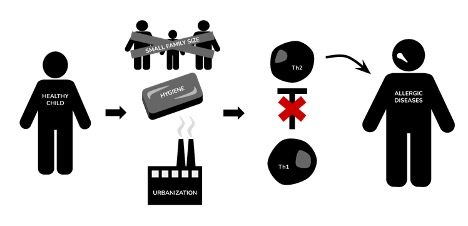
Figure 1: illustrates a simplistic view of the mechanisms of the Hygiene Hypothesis before its evolution to the “Old Friends” Hypothesis. Notably, the previous understanding had many gaps and inaccuracies
Image Source: Created by Author; inspired by Sweere, 2018
Currently, while the “Hygiene Hypothesis” has branched to encompass dozens of related variables including environmental pressure, medication, diet, parasite infection, and others, many researchers have questioned its limiting factors (Alexandre-Silva, 2018). As more research attempts to connect the link between hygiene and microbial exposure, the “Hygiene Hypothesis” is revealed to be increasingly inaccurate. To date, there is no confirmed evidence of the link between personal/home cleanliness and increased risk of allergic disease. Moreover, microbial studies in westernized homes indicate that daily or weekly cleaning habits (even with antibacterial cleaners) have no sustained effect on the levels of microbes in the home (Bloomfield, 2019). Immunologist Sally Bloomfield further notes that Rook’s understanding of excess cleanliness is implausible because of how fast microbes can spread, even after their removal. Thus, this criticism of Strachan’s “Hygiene Hypothesis,” has ushered in the widespread acceptance of Rook’s 2003 “Old Friends” Hypothesis, which observes immune dysregulation through an evolutionary lens.
Mechanisms and Current Understanding of the “Old Friends” Hypothesis
Proposed as an alternative to David Strachan’s “Hygiene Hypothesis,” Graham Rook’s “Old Friends” Hypothesis argues that the vital microbial exposures needed to train the immune system are not the childhood infections that Strachan theorized a decade prior (Bloomfield et al., 2016). Instead, Rook approached pathogen exposure through the lens of evolutionary medicine. The “Old Friends” hypothesis argues that certain microbes co-evolved with humans during primate evolution and in hunter-gatherer societies (Cepon-Robins & Gildner, 2020). Since these microbes had to be tolerated, the immune system responded by developing strategies to activate certain immunoregulatory mechanisms (Alexandre-Silva, 2018). Furthermore, Rook’s hypothesis argues that living with personal “anti-hygienic” habits does not correlate with higher incidences of chronic inflammatory and allergic diseases (Bloomfield et al., 2016). Rook justifies this theory by characterizing most common childhood infections (such as smallpox, tuberculosis, and the flu) as “crowd infections” because they only flourish in large, crowded communities. Rook furthers that because these “crowd infections” came too late into the evolution of the immune system, exposure to these dangerous infectious pathogens is nonessential to building a strong immune system. Instead, the “Old Friends” Hypothesis states that early exposure to a specific group of antigens present during human’s early evolution (also known as “old friends”) is essential for the immune system to properly respond to novel microbes (Bloomfield et al., 2016).
The “Old Friends” Hypothesis further attempts to understand the disproportional rise of allergies and autoimmune diseases in wealthy, urbanized nations as compared to low-income nations. Rook explains that the advancement of medical, hygiene, and sanitation practices has limited the exposure to these “old friends.” This lack of microbial exposure results in immune dysregulation that favors pro-inflammatory pathways, which overreact to benign microorganisms (Cepon-Robins & Gildner, 2020). Thus, the “Old Friends” Hypothesis marks a clear paradigm shift for understanding the correlation between urbanization and wealth and weakened immune system from a lack of microbes in general toward the lack of exposure to specific “old friends,” known as evolutionary mismatch.
The core argument behind Rook’s “Old Friends” Hypothesis is that “old friends” exposure at a young age is critical to developing a functional immune system. The immune system is comprised of two systems of defense: the innate and adaptive systems. Innate immunity is a nonspecific defense that targets all germs and foreign antigens entering the body by initiating an inflammatory response (“The innate and adaptive immune systems,” 2020). Inflammation is broadly defined as the innate immune system’s response to stimuli and acts by removing injurious pathogens and molecules, minimizing impending infection, and beginning the healing process (Chen et al., 2017). However, adaptive immunity takes over when the innate immune system cannot destroy certain pathogens. The adaptive immune system neutralizes pathogens through a humoral response (antibody-mediated immunity) facilitated by B and T cells. Additionally, the adaptive immune system contains memory lymphocytes (Memory B and T cells), which can recognize an antigen introduced during prior infection or vaccination (Janeway et al., 2001). As a result, the adaptive immune system can mount a stronger and faster immune response when exposed to the antigen for a second time.
In the context of the “Old Friends” Hypothesis, it is critical to understand that infants are born with only the innate immune system. Rook compares an infant’s immune system to a computer “with hardware and software, but no data to process” (Rook et al., 2014). As T cells develop in the thymus, which is largest at birth and during the first years of life, the adaptive immune system begins to develop as infants are exposed to environmental and maternal microbiota (Simon et al., 2015). Therefore, microbial exposure is critical to developing adaptive immunity for three reasons. First, exposure to a broad range of organisms builds up a bank of B and T cells that recognize a diverse set of microbes. Increased exposure to benign microbes accelerates the recognition of novel pathogens (Bloomfield et al., 2016). Second, microbial structures (such as peptidoglycans and lipopolysaccharides) taken from the gut maintain background activation of the innate immune system (Rook et al., 2014). Third, microbial exposure plays a critical role in setting up regulatory mechanisms that stop inappropriate inflammatory responses within the adaptive immune system through regulatory T cells (Tregs). When exposed to an environment with more microbes, inflammation appears when appropriately needed, but is shut off when the pathogen is neutralized (Rook et al., 2014). In other words, the immune system must be able to recognize “neutral” or beneficial bacteria to avoid an acute response. Without proper regulation, the immune system begins to attack the host (autoimmune diseases), harmless molecules in the environment (allergic disorders), and intestinal tissues (inflammatory bowel diseases) (Rook et al., 2014). Therefore, microbial exposure during infancy is vital in building a well-regulated immune system that can mount an effective attack against only certain pathogens, while establishing a vast database of B and T cells in the case of future exposure.
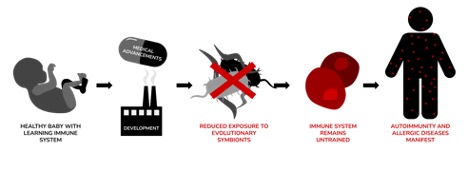
Figure 2: depicts Rook’s idea that evolutionary symbionts train newborn babies’ untrained adaptive immune systems. However, when medical advancements (including antibiotics and Cesarean sections) and development (reducing exposure to the outdoors and increasing sanitary practices) interfere with the infection of these “old friends,” they can experience autoimmunity and allergic diseases when they get older
Image Source: Created by Author
That said, only certain types of “old friends” co-evolved with human immune systems. The first category of these “old friends” is commensal bacteria, which reside on the skin, gut, and respiratory tract (Bloomfield et al., 2016). Both the immune system and commensal microbiota adapt to each other throughout an individual’s lifetime to maintain homeostasis (Khan et. al, 2019). Commensal bacteria prevent the colonization and invasion of harmful bacteria by competing for nutrients and adhesion sites, while simultaneously producing antimicrobial signals to inhibit the growth of these harmful bacteria (Martín et al., 2013).
The second category is found in the environmental microbiota through air, soil, and water. Diminished exposure to these commensal and environmental microbes can enable an increased anti-inflammatory Th2 response, leading to higher incidences of allergy and autoimmune disease (Cepon-Robins & Gildner, 2020). The third category of “old friends” are helminths, parasitic worms that have mutualistically co-evolved with humans in order to survive. These helminths are capable of modulating the host immune system to prevent an effective attack against themselves while blocking other opportunistic species from parasitizing off the host (Alexandre-Silva, 2018). With helminth infections only widespread in poorer countries within Latin American and Africa, researchers can readily compare patients with and without helminthic exposure to further test the “Old Friends” Hypothesis (Alexandre-Silva, 2018).
Beyond helminths, “old infections” (such as variations of Mycobacterium tuberculosis) were also critical to the evolving immune system because they were found in persistent non-fatal carrier states within small isolated hunter-gatherer groups (Rook et al., 2014). This is perhaps where Strachan’s “Hygiene Hypothesis” most sharply differed from Rook’s “Old Friends Hypothesis.” Strachan argued that exposure to conventional childhood infections, such as measles, chickenpox, and mumps was needed to train the immune system. Rook argued that these “crowd infections,” which only flourish in large, centralized populations, were not part of the “human evolutionary experience because they either kill or induce solid immunity” (Bloomfield et al., 2016). Epidemiological research conducted in Finland, Denmark, and the United Kingdom confirm that these infectious diseases do not protect against allergic diseases, making Rook’s “Old Friends” Hypothesis more widely recognized by microbiologists (Bloomfield et al., 2016).
Lastly, Rook’s “Old Friends” Hypothesis proposes how environmental mismatches between hunter-gatherer societies in the Neolithic age and urbanization in a high-income country can limit the exposure to “old friends.” First, the advent of medical, hygiene, and sanitation practices have reduced the presence of “old friends” infections. Most notably, the depletion of historic infections can be seen with the introduction of antibiotics in the 1950s and the subsequent trend toward over prescription (Rook et al., 2014). With antibiotics inducing bacterial cell death, research has found that antibiotics permanently alter the gut microbiota and result in an acute pro-inflammatory immune response (Cully, 2019). This link has been proven in a 2014 review of 50 epidemiological studies that show that excessive antibiotic use, particularly in early childhood, strongly correlates with an increased risk of allergic disease (Bloomfield et al., 2016).
Additionally, the most critical times for “old friends” exposure are early in development, during pregnancy, delivery, and the first few days or months of infancy (Bloomfield et al., 2016). With modern advancements leading toward Caesarean deliveries, research has found that newborns delivered by C-section lack strains of vital gut bacteria and contain harmful opportunistic bacteria that are unique to hospitals (Callaway, 2019). Even the transfer of bacteria through breastfeeding has a large influence on the gut microbiome, and thus shifts in the feeding patterns of infants have long-term implications for the development of allergies (Pannaraj et al., 2019). While more research is needed to confirm any association with allergic disease, key cultural shifts in the treatment of children have resulted in the drastic depletion of the gut microbiome.
Lastly, as cities become more urbanized, infants are in less contact with the animals and green spaces of a “rural microbiome” during the first two-three years of life (Zou et al., 2018). As a result, the gut microbial ecosystem is severely depleted, which is associated with increased allergic sensitization (Rook et al., 2014). Thus, as more humans have drastically shifted their lifestyle from the Neolithic age to the modern urbanized era, the decrease in exposure to microorganisms that co-evolved with humans has resulted in weaker immune responses to foreign antigens.
Effects of Dysbiosis and Evolutionary Mismatch
Given the dependence of adaptive immunity on microorganisms, it should come as no surprise that reduced exposure to symbiotic helminths and bacteria (evolutionary mismatch) and an imbalanced microbiome (dysbiosis) will have consequences. The potential consequences of dysbiosis are plentiful and can arise in many different ways: lifestyle, antibiotic usage, diet changes, and many more. However, we will place emphasis on the outcomes that arise from changes in lifestyle, which are presumably most relevant to the pandemic.
Allergic Diseases
As the original diseases of interest of the Hygiene Hypothesis, the relationship between the “Old Friends” Hypothesis and allergic diseases, including asthma, allergic rhinitis, allergic conjunctivitis, and more, has been extensively studied. Various epidemiological studies reveal that developed countries experience a notable increase in allergic diseases while the same diseases are relatively uncommon in undeveloped countries (Doll et al., 2019). There also appears to be a positive correlation between socioeconomic status and incidence of allergic diseases in Europe, even within the same country. Additional trends can be observed in countries with poor health standards (i.e. less sterilization of food and water, less vaccination, and less antibiotic usage), which exhibit lower incidences of allergic diseases (Okada et al., 2010). As the number of siblings, attendance at daycares, or exposure to farming and livestock all increase, allergic diseases decrease (Okada et al., 2010). Furthermore, since World War II, parasitic infections have nearly been eliminated, and concurrently, allergic disease incidence has increased. In accordance with this trend, certain helminths (Necator americanus and the Schistosoma genus) protect from atopy — the genetic tendency to acquire allergic diseases (Okada et al., 2010).
The “Old Friends” Hypothesis could explain such phenomena. In theory, exposure to commensal microbes through siblings, daycares, and a more rural lifestyle is thought to decrease disease incidence through the aforementioned pathways. Several confounding variables, including better diagnostic methods and increased access to medical clinics (they would both increase the diagnosis of allergic infections even if the incidence remained unchanged), have been identified within these studies. Yet some of the confounding variables, including air pollution levels for asthma, provide insufficient evidence to discredit the “Old Friends” Hypothesis as a possible explanation (Okada et al., 2010). Additionally, several recent studies uphold the “Old Friends” Hypothesis. In both mouse and human models, infection with mycobacteria protects against allergy development. Mycobacteria counteract inflammation and more importantly stimulate Treg responses. Other bacteria, including Lactobacillus and Bifidobacteria species, have been identified to play a protective role in allergy development. Additionally, several studies have found that changes in microbiome population size, composition, and diversity affect allergy incidence (Nordengrun et al., 2018).
Autoimmune Diseases
Type 1 Diabetes (T1D) and Multiple Sclerosis (MS) have been thoroughly studied in the context of the “Old Friends” Hypothesis. T1D and MS exhibit epidemiological trends similar to that of allergic diseases. Decreases in the number of siblings, increases in GDP (a measure of economic productivity), and increases in health standards are associated with increases in autoimmune diseases such as T1D and MS. In Europe, Eastern European countries have lower incidences of T1D than in Western Europe, although incidences are rising in Eastern Europe as its countries develop economically. In the specific case of these autoimmune diseases, this disparity cannot be attributed to advances in diagnostic technology because, unlike allergic diseases, they have been historically easier to diagnose. Differences in genetic backgrounds as a possible confounding variable have also been discredited in the case of T1D and MS. Migration studies reveal that immigrant populations tend to take on the prevalence of autoimmune diseases in the host country, evinced by the increase in T1D in families moving from Pakistan to the United Kingdom and the increase in MS in Asian families moving to the United States (Okada et al., 2010). Additionally, MS patients with parasitic infections presented with improved symptoms and increased transforming growth factor beta (TGF-β), which plays an anti-inflammatory role and stimulates Treg maturation (Okada et al., 2010).
Animal models have shown a causal relationship between an absence of environmental pathogens and diabetes incidence. Non-obese diabetic (NOD) mice were raised in germ-free (GF) conditions, where they exhibited exacerbated diabetes. An accidental contamination of the GF NOD mice with Bacillus cereus resulted in delayed onset and decreased incidence of diabetes, suggesting that specific microbes play a protective role (Gulden et al., 2016). NOD mice bred in specific pathogen free (SPF) environments exhibited a nearly 100% incidence of diabetes. NOD mice bred in environments with diverse bacteria, virus, and parasite composition, in contrast, were protected from diabetes onset (Okada et al., 2010). Many studies investigate the effects of specific strains of bacteria on diabetes incidence. In bio-breeding diabetic-prone (BB-DP) mice, Lactobacillus johnsonii transferred from bio-breeding diabetic-resistant (BB-DR) mice inhibited diabetes onset, while Lactobacillus reuteri had no effect. Segmented filamentous bacteria (SFB) colonization in SPF NOD mice protected from diabetes. However, SFB colonization in GF NOD mice had no effect, illustrating the dependence of SFB’s protective effect on other strains’ presence (Gulden et al., 2016). These studies assert the importance of bacterial diversity, not simply bacterial presence. Patterns of microbiome diversity in T1D patients corroborate such findings as T1D patients have exhibited decreased diversity compared to a healthy control group in certain studies (Gulden et al., 2016).
To study the microbiome’s effects on MS, the murine equivalent of MS, experimental autoimmune encephalomyelitis (EAE), was studied under various conditions. Interestingly, EAE does not occur in GF myelin oligodendrocyte glycoprotein-specific T cell receptor (MOG-TCR) mice who are prone to EAE. When transferred to conventional conditions, the MOG-TCR mice develop EAE. When the GF MOG-TCR mice are recolonized with feces from an MS-affected donor, EAE occurs. When they receive bacteria from a healthy donor, EAE does not occur. GF MOC-TCR mice resistant to autoimmunity do not develop EAE when recolonized with bacteria. Additionally, Clostridia, Bacteroides fragilis, and Lactobacilli all result in symptom improvement by mediating Treg development and increasing anti-inflammatory cytokines (Boziki et al., 2020). Yet, applying the murine model to human MS is difficult as EAE and MS have notable differences. In clinical studies, many have found similarly imbalanced microbiome compositions in MS patients, but there has not yet been evidence to suggest that dysbiosis causes MS (Boziki et al., 2020).
Inflammatory Bowel Diseases
Inflammatory Bowel Diseases (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD), are closely associated with the “Old Friends” Hypothesis. However, proving a definitive causal relationship between dysbiosis and IBDs has been difficult. IBDs mimic the epidemiological trends of T1D and allergic diseases, becoming more prevalent in industrialized countries. These trends also encounter the same confounding variables (Okada et al., 2010). Several studies note that patients with IBDs display a less diverse microbiome, but whether dysbiosis was the cause or effect of inflammation remains unclear. A temporal cohort study revealed that children newly diagnosed with IBDs still produced unbalanced stool samples, implying that dysbiosis precedes prolonged disease and therapy. As IBDs progress, this imbalance grows more pronounced (Ni et al., 2017).
Despite the uncertainty found in observational studies, animal models and the effectiveness of certain treatments indicate that the microbiota plays a significant role in IBDs. GF mice experience decreased epithelial repair and decreased immune function. The use of antibiotics in early childhood has been associated with increased incidence of CD. This may be because certain pathobionts are selected for through antibiotic usage, leading to adverse effects. The restoration of diversity nullifies their pathogenic effects (Ni et al., 2017) as several strains of bacteria have a protective effect. 17 such strains are found in the Clostridia genus, which provide antigens and TGF-β that stimulate the development of Treg cells. It is through this pathway that they have been found to attenuate colitis in mouse models (Atarashi et al., 2013). Probiotics, antibiotics, and fecal microbiota transplantation (FMT) have had varied effects on the outcomes of IBD patients. Diet, however, has consistently improved IBDs outcome. Enteral nutritional therapy (ENT), which supplements nutrition through a nasal feeding tube, consistently reduces inflammation and alters microbiota composition, often towards dysbiosis (Ni et al., 2017). Additionally, treatment with certain helminths, including Necator americanus (the same helminth that prevented atopy) and Trichuris suis, significantly improved symptoms of patients with CD and UC, suggesting that their absence contributed to the pathology (Okada et al., 2010). The ambiguous nature of these findings necessitates further investigation into the relationship between IBDs, dysbiosis, and evolutionary mismatch.
Neurological Disorders
An emerging area of study is the connection between the microbiome and the nervous system. In humans, a striking 90% of serotonin (5-HT) is produced in the gut microbiome (Baganz & Blakely, 2013). This 5-HT is involved in enteric neuron signaling and immune cell signaling, and thus, it plays a role in gut reflexes and immune responses. In GF mice, 5-HT is significantly reduced (Yano et al., 2015). GF mice, when compared to SPF and normal mice, also exhibit an elevated hippocampus pituitary adrenal (HPA) axis stress response to restraints, secreting greater amounts of adrenocorticotropic hormone and corticosterone. When bacteria were reintroduced at an early stage in development, the HPA stress response was somewhat corrected, but it was unaffected when bacterial colonization occurred during later stages, suggesting there is a critical period. In general, earlier recolonization with bacteria resulted in greater degrees of correction as GF pregnant mice that were introduced to bacteria gave birth to fully corrected mice (Sudo et al., 2004). When two strains of mice were cross-colonized, they assumed the behavior of the mice from which the transplanted microbiota originated (Cryan & Dinan, 2012). Several strains of bacteria decrease levels of brain derived neurotrophic factor (BDNF), which plays an important role in neuron maturation. Yet, when in the presence of probiotics, the dampening effect of these strains was nullified, indicating there is a complex synergy within the microbiome. In GF mice, BDNF was under-expressed, and they presented with poor cognition and memory in response to a novel object or a T-maze (Gareau et al., 2011). GF mice also present with decreased hippocampal functional connectivity and increased cell death in the dentate gyrus of the hippocampus, which is involved in memory formation (Scott et al., 2020). This may play a role in the poor memory and cognition observed by Gareau (2011).
In contrast to previous studies, however, several positive changes have been observed in GF mice. They exhibit lower levels of anxiety in mazes, produce higher levels of serotonin (5-HT) in the hippocampus, and experience a delayed decrease in neurogenesis postnatally (Cryan & Dinan, 2012; Scott et al., 2020). Given such contradictions, the relationship between the microbiome composition, chemical activity in the brain, and behavior appears to be very complex. More research is needed to determine the true effects of dysbiosis on neurobiology.
Potential Effects of the Pandemic
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), the causative agent of the 2019 coronavirus disease (COVID-19), is a pathogen that has limited to no evolved immune response in humans (Cepon-Robins & Gildner, 2020). Notably, most underlying health conditions linked with severe COVID-19 infections are associated with pro-inflammatory immune activity, suggesting that an overactive inflammatory response may drive milder cases into fatal ones (Cepon-Robins & Gildner, 2020). Moreover, COVID-19 patients with the most severe outcomes often exhibit an overactive inflammatory response referred to as a “cytokine storm.” A cytokine storm is a sudden acute increase in pro-inflammatory cytokines, resulting in destruction of human tissue and, in some cases, multiorgan failure and death (Ragab et al., 2020). A major cause of morbidity in COVID-19 patients is acute lung injury brought on by a cytokine storm, which in its most fatal form is known as acute respiratory distress syndrome (ARDS). Although the exact mechanism of ARDS in COVID-19 patients is not fully understood, the excessive production of pro-inflammatory cytokines is considered to be one of the major factors (Ragab et al., 2020). Through the lens of the “Old Friends” Hypothesis, the SARS-CoV-2 virus provides a unique opportunity for researchers to understand how previous exposure to “old friends” impacts the production of proinflammatory cytokines and the strength of cytokine storms following infection by SARS-CoV-2.
Before understanding how exposure to “old friends” may modulate the immune response toward SARS-CoV-2, establishing how Th1, Th17, and Th2 cells affect the immune system by producing cytokines is critical for understanding possible mechanisms. Cytokines are chemical messengers that produce specific inflammatory responses to neutralize foreign pathogens, and can be either pro-inflammatory or anti-inflammatory (Berger, 2000). Th1 and Th17 type cells produce proinflammatory cytokines (interferon-gamma or IFN-γ) that kill viruses and bacteria within the cells; however, excessive Th1 and Th17 response can lead to uncontrolled tissue damage and perpetuate autoimmune responses (Berger, 2000). To counteract Th1 cytokine release, Th2-type cytokines (IL-4, IL-5) release Immunoglobulin E (IgE) antibodies that enable an allergic reaction to occur. Concurrently, overexpression of Th2 cytokines leads to diseases involving overly proactive immune systems like allergies and asthma (Berger, 2000).
Here, the role of helminths may once again be relevant. In the context of the COVID-19 pandemic, some researchers hypothesize that soil transmitted helminths (STH) may alleviate the cytokine storm and, thus, lead to better COVID outcomes. Further investigation is still required in order to validate this hypothesis, but as it stands, STHs may provide a key avenue to identify the impact of “old friends” on cytokine storm severity.
Today, STHs infect more than a quarter of the global population infected with ascarids (800 million cases), whipworm (400 million cases), and hookworm (400 million cases) (Cepon-Robins & Gildner, 2020). Additionally, because STHs predominantly impact vulnerable low-income populations with poor sanitation and healthcare access, the prevalence of STHs differs widely between developed and developing nations. (Abdeltawabi et al., 2017).
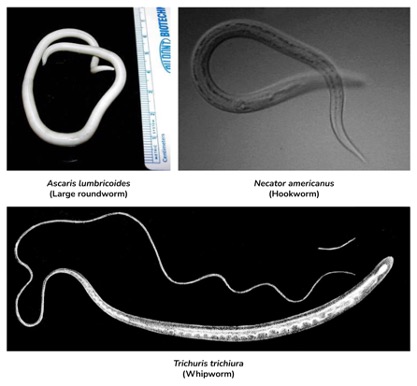
Figure 3: shows three extremely common and important STHs. Each typically results in patients who are asymptomatic or mildly symptomatic
Image Source: Wikimedia Commons
The mechanism of immunomodulation due to immune priming during STH infection has been widely studied. During STH infection, antigens secreted directly from the parasite trigger the anti-inflammatory Th2 response. More specifically, helminth antigens actively suppress inflammation by blocking the Th1 pathway through IL-12 suppression. This helminth immunoregulation ultimately leads to asymptomatic infections favoring the production of anti-inflammatory cytokines (also known as Th2 priming) (Colombo & Grencis, 2020). Additionally, IL-10, IL-4, and IL-13 are critical for host tolerance of helminth infection because IL-10 is anti-inflammatory, while IL-4 and IL-13 are involved in tissue damage repair (Colombo & Grencis, 2020). Most notably, the mechanisms that STHs use to trigger immune tolerance and reduce inflammation are incredibly similar to the mechanisms that humans use to promote tolerance for environmental antigens and commensal bacteria, as well as self-tolerance (Cepon-Robins & Gildner, 2020). Thus, immune priming from STH infection when the immune system is still developing may provide systemic protection from inflammation (Bach, 2018). By skewing future immune responses toward Th2-dominated adaptive immunity, the immune system can become balanced against Th1 immunity and the innate inflammatory response it entails (Cepon-Robins & Gildner, 2020). Unlike helminths, the SARS-CoV-2 virus triggers the proinflammatory Th1 immune response. Elevated levels of pro-inflammatory cytokines IL-6, IL-8, and TNF-alpha are found to be important predictors of COVID-19 severity and patient survival (Del Valle et al., 2020). This suggests that imbalanced immune activity skewed toward the Th1 mechanisms appears to be the primary driver of COVID-19 morbidity and mortality.
Given the immune mechanisms of STHs and SARS-CoV-2, differences between helminth-induced immunoregulation and the typical immune response to SARS-CoV-2 remains critical to understanding whether the “Old Friends” Hypothesis remains pertinent to COVID-19.
Upon entering the body, SARS-CoV-2 infects cells through host ACE2-receptors. The elevated inflammatory response in COVID-19 appears to be associated with an increase in ACE-2 receptor expression. Elevated ACE-2 expression may result in the heightened activation of proinflammatory Th1 and Th17 responses. These pathways inhibit T-reg cell differentiation, which blocks the ability of Tregs to suppress inflammatory cytokines. As these proinflammatory cytokines proliferate uncontrollably, a cytokine storm ensues, leading to the onset of ARDS (Cepon-Robins & Gildner, 2020).
However, helminth-induced immunoregulation has the effect of decreasing ACE-2 receptor expression, and thus, decreases the inflammatory impact of SARS-CoV-2. Additionally, STHs prime the immune system to balance the Th1 and Th17 response with the anti-inflammatory Th2 response. In turn, this produces CD4+ T-cells (helper T-cells) and Treg cells, which enables anti-inflammatory cytokines to react to the virus (Quinn et al., 2019). With proinflammatory Th1 and Th17 pathways more balanced by anti-inflammatory Th2 pathways, STH induced immunoregulation reduces the likelihood of a cytokine storm forming. Therefore, individuals previously infected with STHs may experience reduced viral load, milder COVID-19 symptoms, and no cytokine storm (Cepon-Robins & Gildner, 2020).
It is possible that STH-linked immunoregulation may increase the risk of initial infection, because of the finite capability of the immune system. However, STH-linked immunoregulation may also lead to milder symptoms and reduced mortality, as is shown with coinfection between STHs and malaria (Getachew et al., 2013). The main takeaway is that, given the capacity of STH infection to boost the anti-inflammatory Th2 response, individuals who have been exposed to STHs may experience less severe cytokine storms. If this hypothesis holds true, it means that low-income, non-urbanized communities with endemic STH infections would exhibit reduced severity of SARS-CoV-2. Still, epidemiological studies that compare COVID-19 severity to STH infection (possibly between developed and undeveloped countries) are needed to evaluate the accuracy of this prediction. Despite these gaps in research, Rook’s “Old Friends” Hypothesis provides an invaluable, novel lens to frame possible therapeutic solutions to the ever growing COVID-19 pandemic.
Consequences of “Hygiene Hypothesis” Misconceptions in the Pandemic
With the majority of media propagating the “Hygiene Hypothesis” to the public, dangerous misconceptions about the role of personal hygiene in the development of allergies and autoimmune disorders have played a detrimental role in protecting children’s immune systems (Scudellari, 2017). A survey conducted by the Royal Society for Public Health in June 2019 found that almost one in four (23%) individuals agreed with the statement “hygiene in the home is not important because children need to be exposed to harmful germs to build their immune system” (Royal Society for Public Health, 2019). Especially during the COVID-19 pandemic, where schools shut down and stricter lockdown guidelines are enforced, many parents are increasingly concerned that prolonged COVID-19 safety measures could have a significant impact on their children’s immune system.
These concerns are not entirely unfounded. The significant lifestyle changes may indeed have lasting impact on the immune systems of young children. Considering that exposure to others is minimal, children may miss out on acquiring “old friends” through places with high levels of bacteria (schools, playgrounds, restaurants etc.) As a result, allergies, autoimmune diseases, IBDs, and neurological disorders may become more prevalent. Alternatively, it is also possible that children spending more time outside will restore contact with the “old friends” that reside in parks and nature. However, it is important to note that these claims are purely speculative. No research has yet been published to assert that the strict quarantine measures will significantly impact microbiome composition, let alone children’s immune systems.
Without properly understanding why the “Hygiene Hypothesis” has been surpassed by more successful theories, parents may end up exposing their children to dangerous pathogens (including SARS-CoV-2) with missguided, good intentions. Through the lens of the “Old Friends” Hypothesis, the public perception of being “too clean in our own homes” remains unfounded in the face of current research. An explosion of data obtained using RNA sequencing of samples from US homes suggests that modern homes are “teeming with microbes” (Bloomfield et al., 2016). With studies confirming that effective personal hygiene can kill dangerous pathogens, environmental bacteria from outside and commensal bacteria from other people provide households with microbiota (Bin Abdulrahman et al., 2019). Additionally, Rook’s “Old Friends” Hypothesis argues that “crowd infections,” such as measles and the common cold appeared far too late in our evolutionary history to play a critical role in developing our immune system. Thus, public belief that exposing children to COVID-19 by abandoning lockdown measures and safety guidelines improves immune response puts children in a dangerous position. Instead, making sure that kids are properly vaccinated, have enough time to play outside, and follow COVID-19 safety guidelines have been widely accepted by the scientific community to be the best way to protect the immune health of children (Moyer, 2020).
Conclusion
Since the conception of the Hygiene Hypothesis in 1989, the hypothesis has been transforming. Controversy and novel findings catalyzed its rapid evolution into the “Old Friends” Hypothesis. Now, the idea that human immunity relies on bacterial and parasitic symbionts is becoming more widely accepted. As a result, its relationship with many pathologies is being explored. Most notably, the “old friends” seem to play a significant role in the development of allergic diseases and T1D. Their exact effect on MS, IBDs, and neurological disorders is more uncertain. Each of these diseases has been associated with living in more sterile and urbanized conditions. Interestingly, the pandemic has pushed the American population to live more hygienically and to spend more time outdoors. These drastic lifestyle changes observed during the pandemic may have some effect on the exposure of the population to the “old friends.”
Ultimately, the possibility of the pandemic altering incidences of the aforementioned diseases should be researched. For example, a longitudinal study that compares disease incidence in previous generations of children to current “COVID babies” and young children may provide some useful insight. Researchers can also profile the bacterial composition in stool samples of children who grew up during the pandemic and compare it to existing samples from healthy children. However, it is important to note that, regardless of these attractive research possibilities, adhering to CDC guidelines takes precedence over the speculations presented in this review. The possible benefits of helminth-induced immunoregulation are also highly interesting. Investigating the potential clinical benefits of helminths in the context of SARS-CoV-2 with murine models and clinical trials might present a promising preventative treatment for this virus and others.
References
Alexandre-Silva, G. M., Brito-Souza, P. A., Oliveira, A. C. S., Cerni, F. A., Zottich, U., & Pucca, M. B. (2018). The hygiene hypothesis at a glance: Early exposures, immune mechanism and novel therapies. Acta Tropica, 188, 16–26. https://doi.org/10.1016/j.actatropica.2018.08.032
Atarashi, K., Tanoue, T., Oshima, K., Suda, W., Nagano, Y., Nishikawa, H., Fukuda, S., Saito, T., Narushima, S., Hase, K., Kim, S., Fritz, J. V., Wilmes, P., Ueha, S., Matsushima, K., Ohno, H., Olle, B., Sakaguchi, S., Taniguchi, T., … Honda, K. (2013). Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature, 500(7461), 232–236. https://doi.org/10.1038/nature12331
Bach, J.-F. (2018). The hygiene hypothesis in autoimmunity: The role of pathogens and commensals. Nature Reviews Immunology, 18(2), 105–120. https://doi.org/10.1038/nri.2017.111
Baganz, N. L., & Blakely, R. D. (2013). A Dialogue between the Immune System and Brain, Spoken in the Language of Serotonin. ACS Chemical Neuroscience, 4(1), 48–63. https://doi.org/10.1021/cn300186b
Berger, A. (2000). Th1 and Th2 responses: What are they? BMJ : British Medical Journal, 321(7258), 424.
Bloomfield, S. F., Rook, G. A., Scott, E. A., Shanahan, F., Stanwell-Smith, R., & Turner, P. (2016). Time to abandon the hygiene hypothesis: New perspectives on allergic disease, the human microbiome, infectious disease prevention and the role of targeted hygiene. Perspectives in Public Health, 136(4), 213–224. https://doi.org/10.1177/1757913916650225
Boziki, M. K., Kesidou, E., Theotokis, P., Mentis, A.-F. A., Karafoulidou, E., Melnikov, M., Sviridova, A., Rogovski, V., Boyko, A., & Grigoriadis, N. (2020). Microbiome in Multiple Sclerosis; Where Are We, What We Know and Do Not Know. Brain Sciences, 10(4), 234. PubMed. https://doi.org/10.3390/brainsci10040234
Callaway, E. (2019). C-section babies are missing key microbes. Nature. https://doi.org/10.1038/d41586-019-02807-x
Cepon-Robins, T. J., & Gildner, T. E. (2020). Old friends meet a new foe. Evolution, Medicine, and Public Health, 2020(1), 234–248. https://doi.org/10.1093/emph/eoaa037
Charles A Janeway, J., Travers, P., Walport, M., & Shlomchik, M. J. (2001). Immunological memory. Immunobiology: The Immune System in Health and Disease. 5th Edition. https://www.ncbi.nlm.nih.gov/books/NBK27158/
Chatterjee, B., Karandikar, R. L., & Mande, S. C. (2020). The mortality due to COVID-19 in different nations is associated with the demographic character of nations and the prevalence of autoimmunity. MedRxiv, 2020.07.31.20165696. https://doi.org/10.1101/2020.07.31.20165696
Chen, L., Deng, H., Cui, H., Fang, J., Zuo, Z., Deng, J., Li, Y., Wang, X., & Zhao, L. (2017). Inflammatory responses and inflammation-associated diseases in organs. Oncotarget, 9(6), 7204–7218. https://doi.org/10.18632/oncotarget.23208
Cryan, J. F., & Dinan, T. G. (2012). Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nature Reviews Neuroscience, 13(10), 701–712. https://doi.org/10.1038/nrn3346
Cully, M. (2019). Antibiotics alter the gut microbiome and host health. Nature Research. https://doi.org/10.1038/d42859-019-00019-x
Del Valle, D. M., Kim-Schulze, S., Huang, H.-H., Beckmann, N. D., Nirenberg, S., Wang, B., Lavin, Y., Swartz, T. H., Madduri, D., Stock, A., Marron, T. U., Xie, H., Patel, M., Tuballes, K., Van Oekelen, O., Rahman, A., Kovatch, P., Aberg, J. A., Schadt, E., … Gnjatic, S. (2020). An inflammatory cytokine signature predicts COVID-19 severity and survival. Nature Medicine, 26(10), 1636–1643. https://doi.org/10.1038/s41591-020-1051-9
Doll, R. J., Joseph, N. I., McGarry, D., Jhaveri, D., Sher, T., & Hostoffer, R. (2019). Epidemiology of Allergic Diseases. In Allergy and Asthma: The Basics to Best Practices (pp. 31–51). Springer International Publishing. https://doi.org/10.1007/978-3-030-05147-1_2
Gareau, M. G., Wine, E., Rodrigues, D. M., Cho, J. H., Whary, M. T., Philpott, D. J., MacQueen, G., & Sherman, P. M. (2011). Bacterial infection causes stress-induced memory dysfunction in mice. Gut, 60(3), 307. https://doi.org/10.1136/gut.2009.202515
Getachew, M., Tafess, K., Zeynudin, A., & Yewhalaw, D. (2013). Prevalence Soil Transmitted Helminthiasis and malaria co-infection among pregnant women and risk factors in Gilgel Gibe dam Area, Southwest Ethiopia. BMC Research Notes, 6, 263. https://doi.org/10.1186/1756-0500-6-263
Gülden, E., Wong, F. S., & Wen, L. (2015). The gut microbiota and Type 1 Diabetes. Clinical Immunology (Orlando, Fla.), 159(2), 143–153. PubMed. https://doi.org/10.1016/j.clim.2015.05.013
Khan, R., Petersen, F. C., & Shekhar, S. (2019). Commensal Bacteria: An Emerging Player in Defense Against Respiratory Pathogens. Frontiers in Immunology, 10. https://doi.org/10.3389/fimmu.2019.01203
Martín, R., Miquel, S., Ulmer, J., Kechaou, N., Langella, P., & Bermúdez-Humarán, L. G. (2013). Role of commensal and probiotic bacteria in human health: A focus on inflammatory bowel disease. Microbial Cell Factories, 12(1), 71. https://doi.org/10.1186/1475-2859-12-71
Moyer, M. W. (2020, September 10). Is Staying Home Harming Your Child’s Immune System? The New York Times. https://www.nytimes.com/2020/09/10/parenting/children-immunity-staying-home-coronavirus.html
National Center for Biotechnology Information. (2020). The innate and adaptive immune systems. In InformedHealth.org [Internet]. Institute for Quality and Efficiency in Health Care (IQWiG). https://www.ncbi.nlm.nih.gov/books/NBK279396/
Ni, J., Wu, G. D., Albenberg, L., & Tomov, V. T. (2017). Gut microbiota and IBD: causation or correlation? Nature Reviews Gastroenterology & Hepatology, 14(10), 573–584. https://doi.org/10.1038/nrgastro.2017.88
Nordengrün, M., Michalik, S., Völker, U., Bröker, B. M., & Gómez-Gascón, L. (2018). The quest for bacterial allergens. Infections by Gram-Positive Pathobionts Staphylococcus Aureus and Streptococcus Pneumoniae – from Colonization to Invasive Infections, 308(6), 738–750. https://doi.org/10.1016/j.ijmm.2018.04.003
Okada, H., Kuhn, C., Feillet, H., & Bach, J.-F. (2010). The “hygiene hypothesis” for autoimmune and allergic diseases: An update. Clinical and Experimental Immunology, 160(1), 1–9. PubMed. https://doi.org/10.1111/j.1365-2249.2010.04139.x
Pannaraj, P. S., Li, F., Cerini, C., Bender, J. M., Yang, S., Rollie, A., Adisetiyo, H., Zabih, S., Lincez, P. J., Bittinger, K., Bailey, A., Bushman, F. D., Sleasman, J. W., & Aldrovandi, G. M. (2017). Association Between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome. JAMA Pediatrics, 171(7), 647. https://doi.org/10.1001/jamapediatrics.2017.0378
Quinn, S. M., Kyle Cunningham, Raverdeau, M., Walsh, R. J., Curham, L., Malara, A., & Mills, K. H. G. (2019). Anti-inflammatory Trained Immunity Mediated by Helminth Products Attenuates the Induction of T Cell-Mediated Autoimmune Disease. Frontiers in Immunology, 10. https://doi.org/10.3389/fimmu.2019.01109
Ragab, D., Salah Eldin, H., Taeimah, M., Khattab, R., & Salem, R. (2020). The COVID-19 Cytokine Storm; What We Know So Far. Frontiers in Immunology, 11. https://doi.org/10.3389/fimmu.2020.01446
Rook, G. A. W., Raison, C. L., & Lowry, C. A. (2014). Microbial ‘old friends’, immunoregulation and socioeconomic status. Clinical and Experimental Immunology, 177(1), 1–12. https://doi.org/10.1111/cei.12269
Schaub, B., Lauener, R., & von Mutius, E. (2006). The many faces of the hygiene hypothesis. The Journal of Allergy and Clinical Immunology, 117(5), 969–977; quiz 978. https://doi.org/10.1016/j.jaci.2006.03.003
Scott, G. A., Terstege, D. J., Vu, A. P., Law, S., Evans, A., & Epp, J. R. (2020). Disrupted Neurogenesis in Germ-Free Mice: Effects of Age and Sex. Frontiers in Cell and Developmental Biology, 8, 407. https://doi.org/10.3389/fcell.2020.00407
Scudellari, M. (2017). News Feature: Cleaning up the hygiene hypothesis. Proceedings of the National Academy of Sciences, 114(7), 1433–1436. https://doi.org/10.1073/pnas.1700688114
Simon, A. K., Hollander, G. A., & McMichael, A. (2015). Evolution of the immune system in humans from infancy to old age. Proceedings of the Royal Society B: Biological Sciences, 282(1821). https://doi.org/10.1098/rspb.2014.3085
Stiemsma, L., Reynolds, L., Turvey, S., & Finlay, B. (2015). The hygiene hypothesis: Current perspectives and future therapies. ImmunoTargets and Therapy, 143. https://doi.org/10.2147/ITT.S61528
Strosberg, S. (2014). THE HUMAN–HOOKWORM ASSEMBLAGE: CONTINGENCY AND THE PRACTICE OF HELMINTHIC THERAPY. Theses and Dissertations–Geography. https://uknowledge.uky.edu/geography_etds/21
Sudo, N., Chida, Y., Aiba, Y., Sonoda, J., Oyama, N., Yu, X.-N., Kubo, C., & Koga, Y. (2004). Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. The Journal of Physiology, 558(1), 263–275. https://doi.org/10.1113/jphysiol.2004.063388
Sweere, J. (2018, August 7). Hello Microbe My Old Friend: How a Diverse Microbiome Trains the Immune System Against Allergies. The Dish on Science. http://thedishonscience.stanford.edu/posts/microbe-old-friends-allergies/
von Mutius, E. (2007). Allergies, infections and the hygiene hypothesis—The epidemiological evidence. Immunobiology, 212(6), 433–439. https://doi.org/10.1016/j.imbio.2007.03.002
von Mutius, E. (2010). 99th Dahlem Conference on Infection, Inflammation and Chronic Inflammatory Disorders: Farm lifestyles and the hygiene hypothesis. Clinical and Experimental Immunology, 160, 130–135. https://doi.org/10.1111/j.1365-2249.2010.04138.x
World Wide Epidemiology of Helminths Infection | IntechOpen. (n.d.). Retrieved December 30, 2020, from https://www.intechopen.com/books/human-helminthiasis/world-wide-epidemiology-of-helminths-infection
Yano, J. M., Yu, K., Donaldson, G. P., Shastri, G. G., Ann, P., Ma, L., Nagler, C. R., Ismagilov, R. F., Mazmanian, S. K., & Hsiao, E. Y. (2015). Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell, 161(2), 264–276. PubMed. https://doi.org/10.1016/j.cell.2015.02.047
Zuo, T., Kamm, M. A., Colombel, J.-F., & Ng, S. C. (2018). Urbanization and the gut microbiota in health and inflammatory bowel disease. Nature Reviews Gastroenterology & Hepatology, 15(7), 440–452. https://doi.org/10.1038/s41575-018-0003-z
Related Posts
The I.D.E.A. Initiative: Infectious Disease Educational Awareness
This publication is in proud partnership with Project UNITY’s Catalyst Academy 2023...
Read MoreThermophilic Life in Yellowstone National Park Challenges the Physical and Chemical Limits of Survival
Cover Image: Grand Prismatic Spring in Yellowstone National Park (Source:...
Read MoreThe Ascent of Computational Techniques in Life Science Research
The prominence of computational techniques in the field of research...
Read More