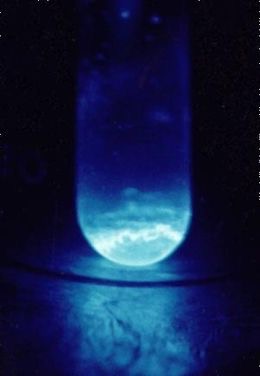
Figure 1: One of the few things that we know about it, einsteinium has a strong luminescence because of its highly radioactive nature. Many of its other physical and chemical properties remain unknown due to its rarity
Source: Wikimedia Commons
In studying the chemical properties of transition metals many properties such as the placement of electrons in atomic orbitals and common oxidation states, or the number of electrons that can be lost to stabilize an element, – remain fairly predictable. However, the actinide series – the range of elements from actinium to lawrencium – have demonstrated some unusual physical and chemical properties due to the diffuse nature of their 5f atomic orbitals, which allows for interesting changes in chemical structure such as having multiple possible oxidation states (Morss et al., 2010). Additionally, while some atoms like uranium and plutonium have had extensive studies done due to their relative abundance, heavier elements up to and beyond Fermium (atomic number 100) have not received as much study due to their relative rarity and high radioactivity (Chao, 2021).
One element that has received particular interest in chemical studies is einsteinium. First discovered in debris from the test of the “Ivy Mike” nuclear weapon in 1952, the 99th element on the periodic table is incredibly rare, with a half-life for most of its isotopes – atoms with the same number of protons but differing numbers of neutrons – existing on the order of minutes to hours (Morss et al., 2010). However, based on previous studies of the element, it is believed that einsteinium has its electrons “localized” in its 5f orbitals, meaning that electrons do not jump from between orbitals at a high rate because of the large energy gap that would need to be overcome between the 6d and 5f orbitals (Morss et al., 2010). Additionally, einsteinium is unique from the other actinide elements – it can have a valence of 2 – meaning that it has two bonding electrons, whereas the other actinide elements have valences of three. This allows einsteinium to reach a +2 oxidation state and have a 5f11 electron configuration, which enables it to form different molecular geometries compared to its counterparts within the same orbital (Hobart, 2011). Because of this interesting phenomenon and associated interesting properties, scientists have sought methods to study and characterize complexes of the metal to understand how it forms chemical bonds. Until recently, the rarity of the metal, only available in nanograms, precluded further studies of its chemical properties (Carter et al., 2021).
However, recent work from Carter et. al, a group out of the Lawrence Berkeley National Laboratory has revealed some of the curious properties of einsteinium using less than 200 nanograms of the metal. The group chose to chelate, or bind a hydroxypyridinone ligand to einsteinium, partly because of the ligand’s ability to form many bonds with the metal but also and because the ligand enabled luminescence measurements of the metal complex (Carter et al., 2021). Additionally, the group chose to use the isotope Einsteinium-254 – (which has one of the longest half-lives for the element at 275 days)to have sufficient time to analyze the metal complex before the sample degraded (Carter et al., 2021). The group did have to overcome some challenges; for instance, to produce the hydroxypyridinone complex they had to purify the sample from a contaminated mixture with the less useful californium-249, and due to a hiatus caused by the COVID-19 pandemic, the team was unable to perform follow-up experiments to further evaluate einsteinium, as the material had decayed by the time they were able to resume work (Chao, 2021).
Using a technique called ‘extended x-ray absorption fine structure,’ the group was able to validate the structure of the complex as having an Es3+ oxidation state, as well as measure the bond length between einsteinium and the oxygen atoms of the hydroxypyridinone. Based on the analysis, the average bond length between einsteinium and oxygen was found to be 2.38 angstroms, or 2.38*10-10 m (Carter et al., 2021). In comparison, the bond lengths between other actinides, such as americium, curium and californium were found to be somewhat larger, ranging from 2.42 to 2.5 angstroms. The group suggested that this difference in bond length could mean that the atomic orbitals of einsteinium have a different energy level ordering, which could allow einsteinium to form unique complexes that the other actinides cannot. (Carter et al., 2021). The group also chose to analyze how the einsteinium cation’s electron arrangement compared to that of the hydroxypyridinone complex using ‘solution phase luminescence spectroscopy’ (Carter et al., 2021). They found that the hydroxypyridinone complex emitted a higher energy photon when an electron transitioned from its first excited energy state back to the ground, or lowest level, energy state, as compared to the elemental Es3+ ion (Carter et al., 2021). According to lead author Rebecca Abergel, this is unusual behavior compared to other actinide elements, where the complexes emit lower energy photons than the pure elements (Kramer, 2021). While the cause of this is unknown, the group is planning to conduct follow up experiments with near-infrared luminescence spectroscopy to better understand why Einsteinium differs from its actinide neighbors (Carter et al., 2021)
Ultimately, Abergel claimed the group’s work on Einsteinium could open the door to research into other super heavy elements, saying additionally that their studies could both better understand trends in chemical behavior and develop new applications for these elements, such as nuclear power production and radiopharmaceuticals (Chao, 2021).
References
Carter, K.P., Shield, K.M., Smith, K.F. et al.Structural and spectroscopic characterization of an einsteinium complex. Nature 590, 85–88 (2021). https://doi.org/10.1038/s41586-020-03179-3
Chao, J. (2021, February 03). Discoveries at the edge of the PERIODIC Table: First Ever measurements of einsteinium. Retrieved February 09, 2021, from https://newscenter.lbl.gov/2021/02/03/discoveries-at-the-edge-of-the-periodic-table-first-ever-measurements-of-einsteinium/
Hobart, D. (2011). Periodic table of Elements: Los Alamos National Laboratory. Retrieved February 09, 2021, from https://periodic.lanl.gov/99.shtml
Krämer, K. (2021, February 04). Half the world’s supply of element 99 used to reveal its Chemical secrets. Retrieved February 09, 2021, from https://www.chemistryworld.com/news/half-the-worlds-supply-of-element-99-used-to-reveal-its-chemical-secrets/4013168.article
Morss, L. R., Edelstein, N., & Fuger, J. (Eds.). (2010).The chemistry of the actinide and transactinide elements. Dordrecht: Springer.
Related Posts
There’s NO Telling What Nitric Oxide Might Bring to Alzheimer’s Disease Research
Figure 1: As Alzheimer’s disease research begins to shift away...
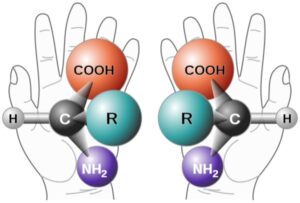
Read MoreSuper Selective Synthesis: The Evolution of Enantioselective Methods
Figure 1: Two enantiomers of an amino acid. These molecules...
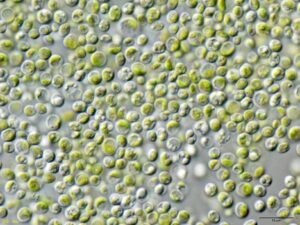
Read More“Living” Droplets Can Produce Renewable Hydrogen
Figure 1: A microscopic image of Chlorella vulgaris, a species...
Read MoreAndrew Sasser

Comments are closed.