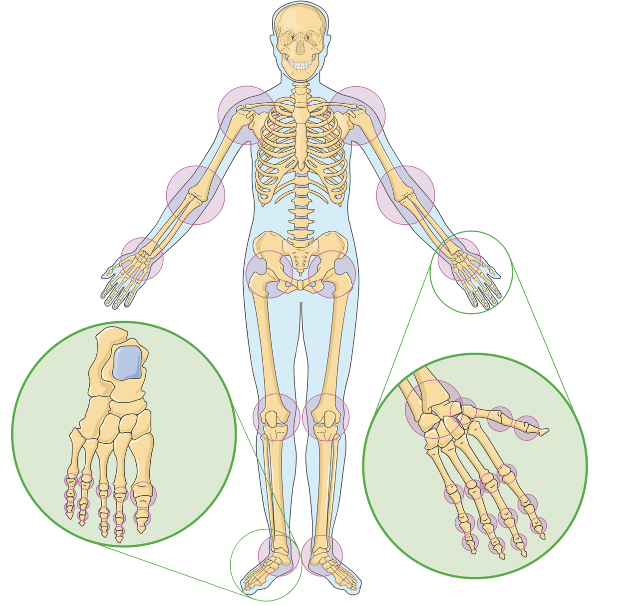
Source: Laboratoires Servier, (CC BY-SA 3.0)
Many of us cannot fathom a life without being able to wiggle our fingers, rotate our shoulders, or jump up and down with ease. However, this is reality for the 250 million people worldwide suffering from osteoarthritis (the degeneration of cartilage at the bone joints), with women and the elderly being the most susceptible1, 2. Although there is no “one-size-fits-all” cure for this condition, some common treatments to relieve symptoms include grafts (tissue transplants) and full joint replacements. However, new tissue that is integrated into the bone-cartilage interface may be rejected by the body or cause the patient trauma4. A major barrier to the development of a definitive cure for osteoarthritis is the intricate nature of the bone-cartilage interface, which, with its multilayered structure and interdependent elements, is still understood relatively poorly by researchers.
Articular cartilage, the specific tissue that covers the ends of bones, lacks the ability to regenerate on its own, leaving patients with damaged cartilage in a very critical condition. However, if scientists are able to promote the production of cartilage cells, they can effectively repair the damaged site. Fortunately, recent research has pointed to an innovative solution capable of just that: 3D bioprinting. The idea is to 3D print a structure that can be loaded with cells and growth factors, which will bind to the damaged site and stimulate cell production. The 3D bioprinting process involves several steps and considerations that are explained below.
Bioink Selection
Bioinks are materials that form the basis of a scaffold – the structure that holds the cells and growth factors being delivered to the body. Due to their high water content, bioinks are able to mimic the properties of the extracellular matrix, the fluid outside of cells that contains important biological molecules. If designed properly, bioinks can solve the issue of the poor biocompatibility of grafts and full joint replacements. However, the two current bioink designs – natural and synthetic – both have their drawbacks. Although natural bioinks have higher biocompatibility, and are less likely to be rejected by the immune system, they have weaker functionality. On the other hand, synthetic bioinks have lower biocompatibility and stronger properties. Therefore, researchers believe that a mix of the two would be fitting for the task at hand3.
Scaffolds
These bioinks are ultimately solidified to create a scaffold, the structural base of the 3D biomaterial. Currently, a scaffold that is not 3D printed may be composed of ceramics, glass, and similar structural materials. However, these compositions should be chosen carefully so that the body does not reject the scaffold. Most importantly, cells must be able to attach to the surface of the scaffold easily. However, the complex nature of the osteochondral interface, particularly its many layers, has complicated the adhesion of traditional scaffolds to the injured area. Scientists have explored the use of bioceramics in scaffold construction, enabling them to create multi-layered scaffolds that mimic the layers of the interface3. Recently, researchers have focused on designing scaffolds to be more biodegradable and more easily deliverable to the body. They have recognized the potential of using bioinks in place of structural materials with poor adhesive abilities to create scaffolds that have a lasting function while simultaneously facilitating essential cell interactions3.
Integration of Cells
The primary function of scaffolds is to carry cells and growth factors that scientists have deemed useful for repairing the damaged site. For example, chondrocytes are critical cells for cartilage formation, as they produce essential proteins that alleviate the stress on joints from compression3. In addition, researchers have paid close attention to the use of MSCs (Multipotent stem cells), which can differentiate into multiple types of cells – chondrocytes and osteoblasts (bone cells) being among them1. The use of patient-derived stem cells would allow scientists to grow cells of their choice and personalize a patient’s treatment. Lastly, growth factors such as kartogenin can further promote chondrocyte production and protection. Before padding these molecules into the bioink structure, they are coated in a special material called hydrogel to protect them while submerged. With all the components in place, the next step is 3D printing.
Bioprinting Methods
Common bioprinting techniques to create a scaffold are extrusion, inkjet bioprinting, and laser-based bioprinting1. Extrusion-based bioprinting uses a nozzle to dispense a constant flow of bioink, which must be solidified quickly so that the structure holds together. This solidification step is done using cross-linking, a technique that holds polymer chains together. In inkjet bioprinting, bioink drips through a nozzle, allowing for greater resolution; however, due to the small size of the nozzle, clogging may occur. Lastly, laser-based bioprinting uses a laser to harden sections of bioink containing light-sensitive substances. Although precise, this approach is very expensive and takes up the most amount of time.
Conclusion
Evidently, 3D bioprinting has significant implications for patients with osteoarthritis, as it can address many of the inherent issues in current therapeutic approaches. Instead of entirely replacing joints, scientists can promote the natural repair of the damaged cartilage by providing it with certain types of growth factors or cells. In addition, scaffold structures can be more biocompatible than grafts and full joint replacements if designed properly, making 3D bioprinting more attractive in the long-term. In the future, research will likely explore techniques to improve scaffolds so that they come even closer to resembling the multi-layered, complex structure of the bone-cartilage interface, optimizing the benefits a patient receives.
References
- Stone, R. N., Reeck, J. C., & Oxford, J. T. Advances in cartilage tissue engineering using Bioinks with decellularized cartilage and three-dimensional printing. International Journal of Molecular Sciences, 24(6), 5526 (2023). https://doi.org/10.3390/ijms24065526
- Centers for Disease Control and Prevention. Osteoarthritis (OA). Centers for Disease Control and Prevention (2020, July 27). https://www.cdc.gov/arthritis/basics/osteoarthritis.htm#:~:text=Age
- Xu, J., Ji, J., Jiao, J., Zheng, L., Hong, Q., Tang, H., Zhang, S., Qu, X., & Yue, B. 3D printing for bone-cartilage interface regeneration. Frontiers in Bioengineering and Biotechnology, 10 (2022). https://doi.org/10.3389/fbioe.2022.828921
- Xue, J., Qin, C., & Wu, C. 3D printing of cell-delivery scaffolds for tissue regeneration. Regenerative Biomaterials, 10 (2023). https://doi.org/10.1093/rb/rbad032
- Ma, K., Zhao, T., Yang, L., Wang, P., Jin, J., Teng, H., Xia, D., Zhu, L., Li, L., Jiang, Q., & Wang, X. Application of robotic-assisted in situ 3D printing in cartilage regeneration with Hama Hydrogel: An in vivo study. Journal of Advanced Research, 23, 123–132 (2020). https://doi.org/10.1016/j.jare.2020.01.010
- Nakamura, A., Murata, D., Fujimoto, R., Tamaki, S., Nagata, S., Ikeya, M., Toguchida, J., & Nakayama, K. Bio-3D printing IPSC-derived human chondrocytes for articular cartilage regeneration. Biofabrication, 13(4), 044103 (2021). https://doi.org/10.1088/1758-5090/ac1c99
Related Posts
According to New Research, Blue Whale Migrations Can Be Studied Through Song
Figure 1: A blue whale’s fluke (tail fin) sticks out...
Read MoreAlleviating Vaccine Hesitancy: The Path to a Safer Future
The human mind is constantly working on the fly, adapting...
Read MoreUniversality of the Face: New Research Points to a Shared Human Commonality in Emotional Expression
Figure 1: Examples of emotional expressions Source: Andrea Piacquadio from Pexels Raising...
Read MoreHow Many Humans Does it Take to Host a Planet?
Figure 1: This is an artist’s rendering of a potential...
Read MoreThermophilic Life in Yellowstone National Park Challenges the Physical and Chemical Limits of Survival
Cover Image: Grand Prismatic Spring in Yellowstone National Park (Source:...
Read MoreThe Public Health Crisis of Alzheimer’s Disease in African American and Hispanic Populations
This publication is in proud partnership with Project UNITY’s Catalyst...
Read MoreSrinitha Sridharan