Figure 1: The human microbiome is the collection of microorganisms that live in and on us and contribute to our health and wellbeing
Source: Leja, 2016
Being amid the COVID-19 pandemic, it would be easy for us to fear all forms of microorganisms. However, not all microbes cause disease and many organisms can actually be beneficial to humans. Microorganisms are constantly surrounding us and inhabiting our bodies. The human body harbors between 10-100 trillion symbiotic microbial cells, a community known as the microbiome (Ursell et al., 2012). Of these, Staphylococcus, a genus of bacteria, is the most common inhabitant present on our skin. Staphylococcus, commonly known as Staph, has a negative or problematic outlook within clinic and hospital care settings due to infection. Yet, most people are unaware that some Staphylococcus species can provide an additional defense barrier against cancer. Experiments have been conducted with the DNA of Staphylococcus epidermidis MO34 which suggest that the bacteria has the capacity to reduce host tumor size due to its production of the molecule 6-N-hydroxyaminopurine (6-HAP). While possessing the MO34 strain of S. epidermidis on your skin may seem like an advantage, that same microbe has also been linked to acne and skin infections when displaced or out of equilibrium. Balance, moderation, and proper conditions are key to a healthy human microbiome, and by using S. epidermidis as an example organism, its beneficial associations with cancer and negative associations with infection can be examined.
S. epidermidis lives in tight association with keratinocytes, the top layer of the human skin. Communication between keratinocytes and the skin microbiota is carried out by microbial biproducts and sensed by the host with dedicated receptors, such as Toll-like receptors (TLPs) (Stacy, 2019). Most microbial products trigger host inflammation, but S. epidermidis is unique as it can decrease host inflammation with TLR2 signaling and promote wound healing. S. epidermidis TLR2 signaling can also reduce inflammation due to Cutibacterium acnes infection, an acne-associated microbe. This chemical production drives communication and favors survival, but some S. epidermidis products have had unexpected, off-target effects, such as 6-N-hydroxyaminopurine (6-HAP). 6-HAP is an antimicrobial that is responsible for the targeting of skin pathogens, but it has recently been shown to inhibit tumor cell growth within hosts.
Strains of S. epidermidis that produce 6-HAP, a molecule that prevents DNA synthesis, are found to selectively inhibit reproduction of tumor cells without interfering with or damaging the normal keratinocytes of skin tissue. To study this finding further, a research team at the University of California-San Diego injected mice with 6-HAP every 48 hours over a two-week period, and the mice showed no signs of toxicity as their weights remained constant (Nakatsuji et al., 2018). When the 6-HAP chemical compound was injected into melanoma cells, the tumor sizes decreased by over 50 percent compared to controls. They found that mice colonized by S. epidermidis capable of producing 6-HAP could reduce UV-induced tumors, unlike mice whose S. epidermidis was incapable of 6-HAP production. This means that an individual possesses a strain of S. epidermidis in their microbiome that produces 6-HAP, they may have more protection against ultraviolet-induced skin cancer than someone whose S. epidermidis is incapable of 6-HAP synthesis. Research is still ongoing to determine exactly which strains produce 6-HAP, but it is known that S. epidermidis MO34 is capable of producing it. Richard Gallo, MD, PhD, Distinguished Professor at UC San Diego School of Medicine also stated that, “Further studies are need to understand how 6-HAP is produced, if it can be used for prevention of cancer or if loss of 6-HAP increases cancer risk,” regarding future areas of research (Galindo, 2018).
A crucial feature of the 6-HAP molecule is that it is capable of being selective with its obstruction of cell growth. 6-HAP activity is controlled by mARCs, mitochondrial amidoxime reducing components (mARC1 and mARC2), which detoxify the enzyme. The expression of mARC genes is much higher in noncancerous keratinocytes compared to cancer cells. To determine if increased mARC expression plays a role in 6-HAP’s selectivity or if another protein is responsible, researchers examined the genes through functional interference. Gene silencing was performed by using siRNA, small RNAs that target mRNA and prevent its translation into protein. After mRNA transcription, instead of the mRNA entering to the ribosome, its correct location for protein synthesis, the siRNA guides the mRNA into the endoribonuclease enzyme complex (RISC) where the mRNA’s message is degraded, and the gene is never expressed (“Gene Silencing,” n.d.). When the keratinocyte’s mARC genes were silenced, the cells were no longer resistant to the 6-HAP molecule’s effects of reducing cell growth. This suggests that the expression of mARC1 or mARC2 is a vital component of 6-HAP resistance in non-cancerous skin cells.
While not every strand of S. epidermidis is capable of 6-HAP production, it is still extremely beneficial microbe to possess, given the ideal conditions. Germ-free mice have been found to be more susceptible to skin infection compared to mice that are kept in specific pathogen-free conditions or are solely associated with S. epidermidis (Nakatsuji et al., 2018). Another example of antimicrobial chemicals produced from S. epidermidis, includes the production of phenol-soluble modulins, which kill bacterial pathogens such as Staphylococcus aureus. According to Nakatsuii et al., evidence has been increasing to show that the skin microbiome plays an important role in the promotion of host defense.
Statistically speaking, just like buying more lottery tickets increases your chance of winning, the more strains of Staphylococcus you have on your skin must increase your chance of 6-HAP production, right? Not exactly. Balance within the human microbiome is essential. What was once a beneficial microbe capable of preventing cancer, can turn into a harmful pathogen if the equilibrium becomes disturbed. Certain Staphylococcus strains can act as opportunistic pathogens – meaning that they don’t infect healthy hosts, but once the microbe becomes displaced or the host becomes ill, the microbe takes its chance to attack and infection becomes present.
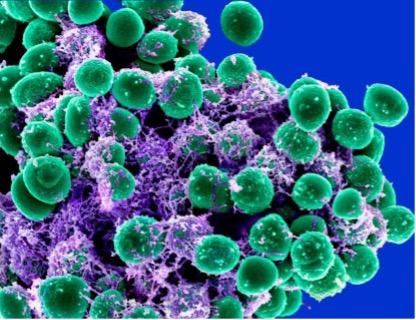
Figure 2: Electron micrograph of Staphylococcus epidermidis bacteria (green) in an extracellular matrix shown in purple, which connects the cells and tissue
Source: NIAID, 2011
S. epidermidis is capable of forming biofilms on surfaces, seen in Figure 2 (NIAID, 2011). Once attached to the skin-surface of the host, S. epidermidis uses its polysaccharide intercellular adhesion (PIA) and its Poly-N-acetylglucosamine (PNAG) to form a biofilm matrix. The PIA and PNAG complexes are responsible for facilitating communication between the staphylococcal cell wall and the biofilm extracellular matrix. The PIA compound is produced by the icaADBC operon, which is a set of linked genes responsible for protein synthesis. Each membrane protein (IcaA, IcaC, and IcaD) produced by the operon has an individual function, as does the IcaB protein that is attached to the staphylococcal cell surface.
IcaA (a N-acetylglucosaminyltransferase, a key enzyme in glycoprotein biosynthesis) and IcaD speed up the conversion of UDP-N-acetylglucosamine into oligomers (10-20 submits in length) of β-1,6-linked poly-N-acetylglucosamine (PNAG). The purpose of the IcaC protein is to extend the N-acetylglucosamine to produce a longer biofilm strand, and the IcaB protein is responsible for controlling the location of PIA on the S. epidermidis cell surface by performing deacetylation, the removal of acetyl groups. S. epidermidis biofilms are more protected than free-floating S. epidermidis from phagocytosis of effector cells, an immune system defense mechanism, but thankfully, not every strain of S. epidermidis contains the ica operon within their genome. Most commensal strains of S. epidermidis are missing one of the icaADBC genes required for the potentially pathogenic operon (Sabaté Brescó et al., 2017).
Having healthy skin is a balancing act between Staphylococcus epidermidis and Cutibacterium acnes. Both of the microbes are present on our skin. In healthy skin, S. epidermidis prevents controls the over-colonization of C. acnes, which contributes to acne (Claudel et al., 2019). If someone struggles with acne, one might assume that getting rid of C. acnes entirely may be the answer to their problems. However, a disequilibrium in favor of S. epidermidis also may result in negative consequences like a nosocomial infection. Therefore, harmony among the skin microbiome is a fundamental component of healthy skin homeostasis.
The balanced presence of life on our skin is essential for the health of our bodies. The human microbiome is beyond fascinating – each person’s microbial community is as distinct as their fingerprint. Due to the COVID-19 pandemic, the public has had an increased interest in the microbial world, and the word “bacteria” is often only seen in a negative connotation. However, it should be emphasized that bacteria are involved in more than just causing disease. With 70% of skin cancer reported being caused by repeated exposure to the sun’s UV rays, it is possible that S. epidermidis strains may reduce, or even prevent cancer growth by producing 6-HAP. By using S. epidermidis as an example organism and examining its associations with cancer and infection, the microbe can be either beneficial or harmful depending on its host conditions and the microbial strains present.
References
Claudel, J. P., Auffret, N., Leccia, M. T., Poli, F., Corvec, S., & Dréno, B. (2019). Staphylococcus epidermidis: A Potential New Player in the Physiopathology of Acne? Dermatology, 235(4), 287–294. https://doi.org/10.1159/000499858
Galindo, Y. (2018). Beneficial Skin Bacteria Protect Against Skin Cancer. https://ucsdnews.ucsd.edu/pressrelease/beneficial_skin_bacteria_protect_against_skin_cancer.
Gene Silencing. (n.d.). Retrieved March 08, 2021, from https://www.ncbi.nlm.nih.gov/probe/docs/applsilencing/
Le, K., Park, M., & Otto, M. (2018). Immune evasion mechanisms of Staphylococcus epidermidis biofilm infection. Frontiers in Microbiology, 9, 359. doi.org/10.3389/fmicb.2018.00359
Leja, D. (2016). Microbiome [Photograph]. Trends in Microbiology. https://www.flickr.com/photos/genomegov/with/27861690225/
Nakatsuji, T., Chen, T. H., Butcher, A. M., Trzoss, L. L., Nam, S. J., Shirakawa, K. T., Zhou,
W., Oh, J., Otto, M., Fenical, W., & Gallo, R. L. (2018). A commensal strain of Staphylococcus epidermidis protects against skin neoplasia. Science advances, 4(2).
https://doi.org/10.1126/sciadv.aao4502
NIAID. (2011). Staphylococcus epidermidis Bacteria [Photograph]. https://www.flickr.com/photos/niaid/5613984108
Sabaté Brescó, M., Harris, L. G., Thompson, K., Stanic, B., Morgenstern, M., O’Mahony, L., … Moriarty, T. F. (2017). Pathogenic Mechanisms and Host Interactions in Staphylococcus epidermidis Device-Related Infection. Frontiers. https://www.frontiersin.org/articles/10.3389/fmicb.2017.01401/full.
Stacy, A., & Belkaid, Y. (2019). Microbial guardians of skin health. Science. https://science.sciencemag.org/content/363/6424/227.full.
Ursell, L., Metcalf, J., Parfrey, L., & Knight, R. (2012). Defining the human microbiome. Nutrition Reviews, 70(1), S38-S44. doi.org/10.1111/j.1753-4887.2012.00493.x
Related Posts
The Next Source of Antibiotics: Viruses
Figure 1: A computer-generated image of a bacteriophage called a...
Read MoreWho gets the Golden Ticket? Navigating the Murky Waters of COVID-19 Vaccine Allocation
Figure 1: The race for a vaccine is in fully...
Read MoreThe E.C.H.O. Initiative: Encouraging Cardiovascular Health Through Outreach
This publication is in proud partnership with Project UNITY’s Catalyst Academy 2023...
Read MoreAnna Schwenn