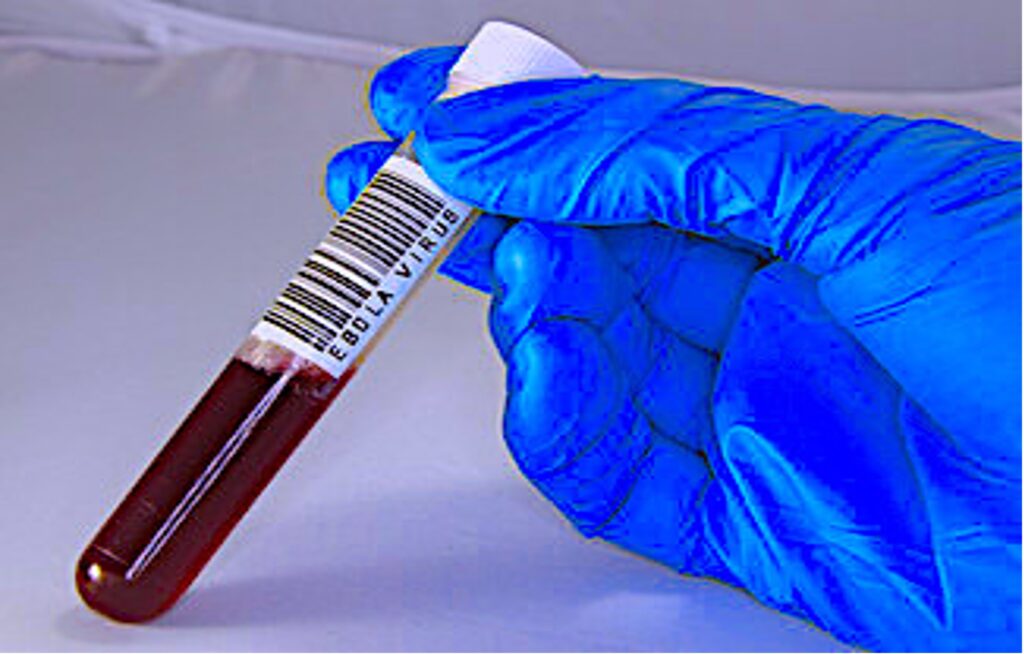
Cover Image: Blood sample of a patient infected by the Ebola virus. (Source: Public Domains Pictures, ChiccoDodiFC)
Ebola virus disease (EVD) is a severe, often lethal infection caused by Ebola virus. Ebola victims present with symptoms such as an acute hemorrhagic fever, severe headache, weakness, and fatigue (Jacob et al., 2020). EVD epidemics typically start with a single case of probable zoonotic transmission (wildlife to human) followed by human-to-human transmission (Groseth et al., 2007). The first recorded Ebola human outbreak took place in 1976 in Sudan when an individual came into contact with the blood of a guinea pig infected by the Ebola virus (EBOV) (Émond RT et al., 1977). The virus simultaneously spread to Zaire, which is now called the Democratic Republic of the Congo. During these outbreaks, 284 cases and 318 cases were confirmed in Sudan and Zaire, respectively. The Sudan strain (SEBOV) had a Case Fatality Rate (CFR) of 53% – meaning that of all the individuals known to be infected, 53% passed away. The Zaire strain (ZEBOV) had an even higher CFR of 89% (Groseth et al., 2007).
Subsequent outbreaks of EBOV revealed the presence of other strains, indicating the successful replication and mutation of the virus. The Reston ebolavirus was genetically discovered in 1989 in the United States of America, brought by Macaques imported from the Philippines; however, it was proven to be non-infectious to humans (Miranda et al, 1996). In 1994, scientists discovered the Ivory Coast Ebola virus (ICEBOV) with a CFR that is less than 1%, and in 2007, another strain was found in Bundibugyo, Uganda (BEBOV) with a CFR of 26% (Muyembe-Tamfum et al., 2012). From all the EBOV species, ZEBOV is the most common and highly lethal strain with 30,706 confirmed cases as of 2021 with an average lethality rate between 25% and 90% (WHO, 2021). Known sources of most EBOV human infections have come primarily from either contact with dead or butchered wildlife, such as apes and chimpanzees, or by transmission to humans in environmental sites that house bats (Groseth et al., 2007; Émond RT et al., 1977).
EVD is contracted from direct contact with bodily fluids (urine, tears, semen, blood, saliva, sweat, vomits, breast milk, feces, etc.) of infected live or dead humans or animals (Figure 1) and makes its way into the body through the skin and mucous membranes (Beeching et al., 2014). It takes about 2-21 days (7 days on average) after infection before the onset of symptoms. Humans become infectious after they develop symptoms (WHO, 2021). Early symptoms may include acute fever, headache, fatigue, vomiting, and muscle pain (Beeching et al., 2014). The late stages of the disease often include multi-organ dysfunction, which is the major cause of death of patients infected by EBOV strains with CFRs ranging between 30% and 90% (WHO, 2021).
Given the virulence and high mortality rate of patients with EVD, in 2014 the World Health Organization declared the Ebola epidemics to be public health emergencies after the deadliest outbreak thus far in Western Africa. This declaration was made to encourage further prevention efforts to keep the virus from disseminating across the globe, and to spur on scientists to develop an understanding of the mechanisms of the EBOV (WHO, 2021).
One field of Ebola research has focused on the characteristics of zoonotic transmission. Researchers have attempted to identify the reservoir species, or natural hosts, of EBOV— i.e., animals that can carry the virus without exhibiting clinical symptoms. Different studies suggest that bats are reservoir hosts of EBOV because the virus was successfully isolated from different bat species with no clinical signs of the illness (Pourrut et al., 2005). Nonetheless, further research needs to be conducted to confirm the theory. Studies to determine the virus reservoirs are important to understand how the virus emerged and to develop risk reduction measures to prevent future EBOV outbreaks.

Figure 1: Ebola virus can be transmitted from several probable reservoirs host to susceptible individuals. (Source: Flickr, James Cridland)
Another scientific quest is to uncover the viral genetic footprints that contribute to the virulence (disease-causing capacity) of EBOV, particularly the ZEBOV strain since it has a higher lethality rate. The strain’s severity is largely due to its remarkable ability to interfere with the host immune response (Wang et al., 2019). ZEBOV inhibits the expression of genes involved in the innate immune response against viral infections, such as the Interferon Regulatory Factor 3 (IRF3) gene, which is an important transcription factor promoting induction of early antiviral immunity (Hartman et al., 2008). EBOV is an RNA virus with a genome that encodes eight viral proteins, including four structural proteins: viral protein 35 (VP35), VP40, VP24, and nucleoprotein (NP) (Wawina-Bokalanga et al., 2019). To produce these proteins, the virus hijacks the host’s cellular machinery – using the same molecular machinery that host cells use to create proteins from their own DNA. VP35 is crucial for EBOV antagonism of interferons, signaling proteins released by the host cell in response to viral infections to elicit an immune response (Hartman et al., 2008). Specifically, VP35 inhibits the activation of IRF3 gene by impairing or blocking IRF3 phosphorylation — an important biochemical process that regulates protein function and signal transmission throughout the cell (Hartman et al., 2008).
Knowing that VP35 is a key factor in the viral attack provides useful information. Scientists have found that the C terminus of the VP35 protein in particular is responsible for its immunosuppressive capacity (Hartman et al., 2008). Therefore, environmental- or human-induced mutations at a specific position on the IRF3 inhibitory domain could considerably decrease the ability of VP35 to act as an interferon antagonist or to silence the IRF3 gene (Hartman et al., 2008).
Scientists have also been looking for environmental and biological patterns that explain the repeated resurgence of EBOV. Researchers have demonstrated that environmental changes or seasonal patterns contribute to the preservation of EBOV in nature (Groseth et al., 2007). One of the scenarios that explains the resurgence of EBOV is that the virus is asymptotically harbored by reservoir species and arises seasonally depending on fitting environmental conditions. Using geographical modeling, Allison Groseth and colleagues found that ZEBOV, ICEBOV, and SEBOV occupy different geographical areas (2007). ZEBOV and ICEBOV outbreaks happened in dry seasons whereas SEBOV outbreaks occurred during the seasonal periods of wetness. This observation coincides with the data from ZEBOV outbreaks in 1996 and 1997 in Gabon whereby a high number of dead great apes was recorded as a result of ZEBOV infection among the population between November and February—a period that marks the dry season in Gabon (Groseth et al., 2007). Another scenario that might explain the recurrence of EBOV in human populations could be the persistence of the virus in some bodily fluids even after complete clinical recovery – although there is not enough evidence at present to know how likely recovered individuals are to transmit the virus (Dukobu et al., 2018).
Many treatments and vaccines against EBOV are in development with the aim of reducing the severity of the disease and preventing future outbreaks. Those vaccines utilize different biological platforms such as DNA vaccines and recombinant viral vectors (Ehrhard et al., 2019; Metzger et al., 208)
Ebola virus is undoubtedly one of the deadliest, known viruses in human history. However, clinical advancements to reduce the severity of the virus are advancing at a satisfactory rate. Nonetheless, the question of whether the world will witness another resurgence of Ebola virus cannot be answered since the origin of the virus is not fully understood.
References
Beeching, N. J., Fenech, M., & Houlihan, C. F. (2014, December 10). Ebola virus disease. The BMJ. https://doi.org/10.1136/bmj.g7348.
Dokubo, E. K., Wendland, A., Mate, S. E., Ladner, J. T., Hamblion, E. L., Raftery, P., Blackley, D. J., Laney, A. S., Mahmoud, N., Wayne-Davies, G., Hensley, L., Stavale, E., Fakoli, L., Gregory, C., Chen, T.-H., Koryon, A., Allen, D. R., Mann, J., & Fallah, M. P. (2018, July 23). Persistence of Ebola virus after the end of Widespread transmission in LIBERIA: An outbreak report. The Lancet Infectious Diseases. https://www.sciencedirect.com/science/article/pii/S1473309918304171.
Ehrhardt, S. A., Zehner, M., Krähling, V., Cohen-Dvashi, H., Kreer, C., Elad, N., Gruell, H., Ercanoglu, M. S., Schommers, P., Gieselmann, L., Eggeling, R., Dahlke, C., Wolf, T., Pfeifer, N., Addo, M. M., Diskin, R., Becker, S., & Klein, F. (2019, October 7). Polyclonal and convergent antibody response to Ebola virus vaccine rVSV-ZEBOV. Nature News. https://www.nature.com/articles/s41591-019-0602-4.
Emond, R. T., Evans, B., Bowen, E. T., & Lloyd, G. (1977, August 27). A case of ebola virus infection. The BMJ. https://www.bmj.com/content/2/6086/541.short.
Groseth, A., Feldmann, H., & Strong, J. E. (2007, August 15). The ecology of ebola virus. Trends in Microbiology. https://www.sciencedirect.com/science/article/abs/pii/S0966842X07001503.
Hartman, A. L., Bird, B. H., Towner, J. S., Antoniadou, Z.-A., Zaki, S. R., [email protected], S. T. N., Amy L. HartmanSpecial Pathogens BranchView all articles by this author, Brian H. Bird University of California, D., Jonathan S. TownerSpecial Pathogens BranchView all articles by this author, Zoi-Anna AntoniadouInfectious Disease Pathology Branch, D. of V. and R. D., Sherif R. ZakiInfectious Disease Pathology Branch, D. of V. and R. D., & Stuart T. Nichol [email protected]Special Pathogens BranchView all articles by this author. (2008, March 15). Inhibition of irf-3 activation by vp35 is critical for the high level of virulence of ebola virus. Journal of Virology. https://journals.asm.org/doi/10.1128/JVI.02344-07.
Jacob, S. T., Crozier, I., Fischer, W. A., Hewlett, A., Kraft, C. S., Vega, M.-A. de L., Soka, M. J., Wahl, V., Griffiths, A., Bollinger, L., & Kuhn, J. H. (2020, February 20). Ebola virus disease. Nature News. https://www.nature.com/articles/s41572-020-0147-3.
Miranda, M. E., Ksiazek, T. G., Retuya, T. J., Khan, A. S., Sanchez, A., Fulhorst, C. F., Rollin, P. E., Calaor, A. B., Manalo, D. L., Roces, M. C., Dayrit, M. M., & Peters, C. J. (1999, February 1). Epidemiology of Ebola (Subtype Reston) virus in the PHILIPPINES, 1996. OUP Academic. https://academic.oup.com/jid/article-abstract/179/Supplement_1/S115/880028.
Muyembe-Tamfum, J. J., Mulangu, S., Masumu, J., Kayembe, J. M., Kemp, A., & Paweska, J. T. (n.d.). Ebola virus outbreaks in Africa: Past and present. Onderstepoort Journal of Veterinary Research. http://www.scielo.org.za/scielo.php?pid=S0030-24652012000200003&script=sci_arttext&tlng=es.
Pourrut, X., Kumulungui, B., Wittmann, T., Moussavou, G., Délicat, A., Yaba, P., Nkoghe, D., Gonzalez, J.-P., & Leroy, E. M. (2005, May 16). The natural history of Ebola virus in Africa. Microbes and Infection. https://www.sciencedirect.com/science/article/pii/S1286457905001437.
S;, M. W. G. V.-M. (n.d.). Questionable efficacy of the rvsv-zebov ebola vaccine. Lancet (London, England). https://pubmed.ncbi.nlm.nih.gov/29565013/.
Wang, W., Wu, C., Amarasinghe, G. K., & Leung, D. W. (2019, May 30). Ebola virus replication stands out. Trends in Microbiology. https://www.sciencedirect.com/science/article/pii/S0966842X19301271.
Wawina-Bokalanga, T., Vanmechelen, B., Martí-Carreras, J., Vergote, V., Vermeire, K., Muyembe-Tamfum, J.-J., Ahuka-Mundeke, S., https://orcid.org/0000-0002-4571-5232, P. M., Tony Wawina-BokalangaKU Leuven, D. of M., Bert VanmechelenKU Leuven, D. of M., Joan Martí-CarrerasKU Leuven, D. of M., Valentijn VergoteKU Leuven, D. of M., Kurt VermeireKU Leuven, D. of M., Jean-Jacques Muyembe-TamfumInstitut National de Recherche Biomédicale (INRB), K., Steve Ahuka-MundekeInstitut National de Recherche Biomédicale (INRB), K., & Piet Maes https://orcid.org/0000-0002-4571-5232KU Leuven, D. of M. (2019, May 16). Complete genome sequence of a New Ebola virus strain isolated during the 2017 Likati outbreak in the Democratic Republic of the Congo. Microbiology Resource Announcements. https://journals.asm.org/doi/abs/10.1128/mra.00360-19.
World Health Organization. (n.d.). Ebola virus disease. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/ebola-virus-disease.
Related Posts
The Connections Between Sleep and Memory
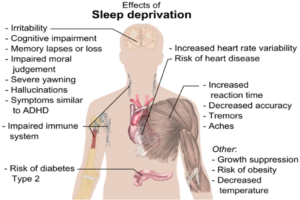
Figure: The image above displays the effects of sleep deprivation...
Read MoreThe Positives of Plushies: Stuffed Animals Have Benefits for Children and Adults
Figure: A young girl sleeps with a teddy bear in...
Read MoreThe Importance of Model Organisms
Many important discoveries in human biology that affect how we...
Read MoreTrust: The Essence of Good Policy Implementation
Figure 1 President Tsai Ing-wen inspects the Central Epidemic Command...
Read MoreThe Neurobiological Basis of Autism
For more neuroscience news, check out the UnknownNow, a student...
Read MoreInvestigating Obstructive Sleep Apnea (OSA) in African and Latin Americans from Chicago
This publication is in proud partnership with Project UNITY’s Catalyst Academy 2024...
Read MoreLord Charité Igirimbabazi