Lead Author: Jillian Troth1
Co-Authors (alphabetical order): Roxanna Attar2, Julia Gainski3, Vincent Lai4, Grace Lee5, Callie Moody1, Megan Nelson6
1Dartmouth College, 2University of Texas at Austin, 3University of Illinois at Urbana-Champaign, 4Rice University, 5Williams College, 6Barrett Honors College at Arizona State University
Cover Image: A fluorescently-stained image of a three-dimensional culture of human breast cancer cells. (Source: Flickr, National Cancer Institute for Cancer Research, NIH)
Introduction
At the most fundamental level, cancer describes a group of related diseases involving the uncontrolled proliferation of abnormal cells which can spread throughout the body (WHO, 2021). Being a major cause of death worldwide, cancer is difficult not only to treat, but also to study (WHO, 2021). In an American Society of Clinical Oncology survey examining the opinions of oncologists from 25 countries, Slovenian scientist Bostjan Seruga and colleagues discovered that obstacles to conducting academic clinical cancer research include a lack of funding, a lack of time, and competing priorities (2014). Low enrollment in cancer clinical trials further complicates research efforts. Illustrative of these problems is the cross-sectional population-based analysis conducted at the Yale School of Medicine that followed participants in a series of therapeutic nonsurgical cancer clinical trials, revealing that enrollment in cancer trials was low across all studied patient groups, with certain groups, such as ethnic minorities, women, and the elderly, being especially unlikely to enroll (2004).
Despite these issues, cancer research has progressed nonetheless in recent decades. Recently, scientists have turned to studying cancer through the lens of the microbiota-gut-brain axis (MGBA), the connection between the gastrointestinal (GI) tract and the brain— itself a growing field of scientific research (Cryan et al., 2019). It has been found that through the MGBA, the microbes living in the gut can influence their host’s health and behavior, including the development and manifestation of cancer (Cryan et al., 2019). For instance, there has been evidence to suggest that gut dysbiosis, or an imbalance of bacteria in the gut, plays a role in cancer progression (Ge et al., 2021). In fact, many possible mechanisms link the gut microbiota to cancer, such as certain inflammatory or immunosuppressive processes, one being the activation of the NF-kB pathway in the colon (Ge et al., 2021; Lawrence, 2009). Additionally, there have been reports that differences in the composition of the gut microbiota are associated with different psychoneurological symptoms in cancer patients undergoing treatment (Song and Bai, 2021). Given these findings, scientists have concluded that it’s plausible the MGBA is involved in cancer pathology, and thus must be considered when studying the onset and development of the illness.
Throughout the course of this article, we will explore the potential role of the MGBA in multiple cancers, highlighting its influence on pathogenesis and on how well patients respond to treatment. Along with cancers that are directly related to the MGBA such as colon and brain cancers, we will also explore how MGBA dysfunction may influence cancers that are indirectly related. Finally, we will briefly discuss MGBA-related cancer treatments that have the potential to replace traditional strategies (such as chemotherapy or radiation) that have historically been very damaging to patient health due to their dangerous side effects.
Colorectal cancer (CRC) — also known as colon cancer — affects 4-5% of the world population, making it one of the most common cancers in the world (Marmol et al., 2017). The main demographic risk factors for colon cancer are age and gender, with males and patients over the age of 65 being at higher risk (Rawla et al., 2019). However, due to increased CRC screening among this age group (and specifically the male subset of this age group), incidence rates have been decreasing for patients over the age of 50. Meanwhile – likely due to the development of more sedentary lifestyles – incidence rates have been increasing among younger populations over the past few decades (Rawla et al., 2019).
Colon Cancer
Studies have shown that a sedentary lifestyle, obesity, and an inflammatory diet are risk factors for CRC as they can influence the gut microbiota and thereby affect intestinal inflammation (Rawla et al., 2019). As such, CRC is more prevalent in developed countries as compared to developing countries, perhaps due to a greater prevalence of such differences in lifestyle and eating habits (Ahmed, 2020). In fact, an increase in intestinal inflammation can cause modification to the gut microflora such that it promotes an immune response, leading to the proliferation of mucosal epithelial cells, or ‘polyps’ (Rawla et al., 2019). Polyps can grow further as time passes, becoming cancerous and progressing to ‘adenocarcinoma,’ a type of colorectal cancer (Rawla et al., 2019).
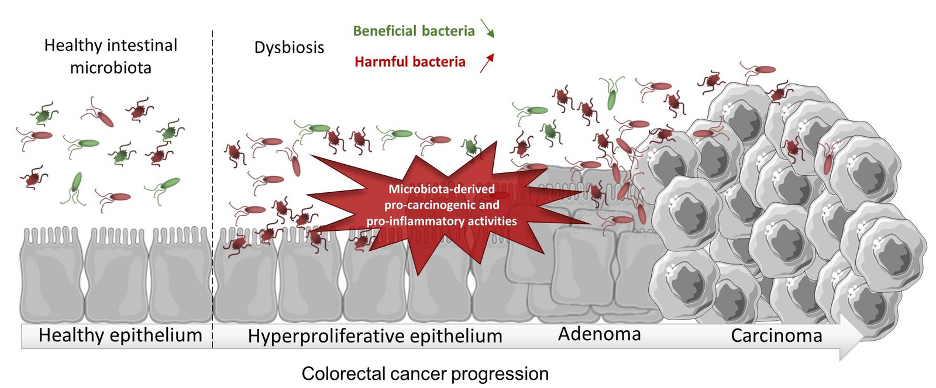
Figure 1: Gut dysbiosis may promote colorectal inflammation, leading to colorectal cancer development. (Source: Wikimedia Commons, Cécily Lucas, Nicolas Barnich and Hang Thi Thu Nguyen).
Polyps with higher abnormalities and a more cancerous appearance are said to have ‘high dysplasia’, or a higher presence of abnormal cells within a tissue (Di et al., 2019). Dysplasia is influenced by the inflammatory state of the gut— and with more inflammation comes greater dysplasia (Colucci et al., 2003). A consequence of higher dysplasia is the disruption of the intestinal epithelia and mucosal barrier, allowing the gut to be more permeable to bacteria and exposing those bacteria to immune gatekeepers known as antigen presenting cells (APCs) (Hasstedt, 2019). A leaky gut thereby creates a positive feedback loop where leakage of bacterial antigens leads to further immuno-inflammatory responses. Specifically, intestinal inflammation can be exacerbated by the gut microbiota through the activation of the NF-kB pathway, a pathway that produces pro-inflammatory chemicals called cytokines, encourages the proliferation of intestinal epithelial cells, and stimulates the growth of CRC tumors toward a more advanced stage (Lawrence, 2009).
Greater cytokine release can also affect the brain because it increases the permeability of the blood brain barrier, modulating brain function and leading to the development of anxiety, depression, and memory loss (Hasstedt, 2019). Additionally, due to their higher incidence of severe inflammation and dysbiosis, patients with inflammatory bowel disease have a much greater chance of developing CRC (Hasstedt, 2019). Studies have also shown that patients with both IBD and CRC overexpress genes that are regulators in the NF-kB pathway, so it seems plausible that the gut microbiome and the MGBA have direct effects on the pathogenesis of colon cancer (Hasstedt, 2019)
Brain Cancer
Brain cancer constitutes a rapid growth of abnormal cells in the brain, with primary brain tumors beginning in the brain, and secondary (or metastatic) brain tumors beginning in other areas of the body and traveling to the brain (Mayo Clinic Staff, n.d.). The time it takes a brain tumor to develop varies generally by ‘grade’ (a description of the cancer cells’ abnormality), while the growth rate and location of the tumor determine the cancer’s impact on nervous system function (Mayo Clinic Staff, n.d.; National Cancer Institute, 2013). Symptoms and signs of brain cancer may include issues with balance, vision, speech, and hearing, as well as an onset of seizures, nausea and vomiting, and patterns of potentially worsening headaches (Mayo Clinic Staff, n.d.).
When doctors construct a treatment plan for brain cancer, they take into account the type and grade of the tumor; the faster the tumor growth, the higher grade the tumor receives and the worse the prognosis (American College of Surgeons, 2017). Additional factors in determining prognosis include histology (specifically tumor type and grade), the patient’s age and symptoms, the extent of ‘tumor residual’ (the remaining tumor post-surgery), tumor location, molecular features, functional neurological status, metastatic spread, and whether or not the tumor is recurrent (American College of Surgeons, 2017).
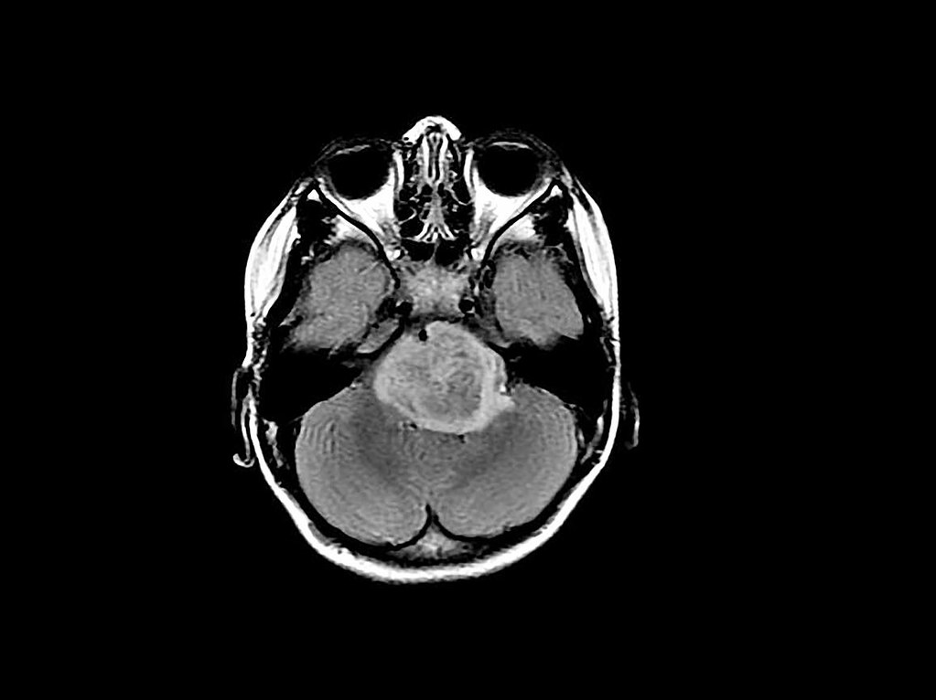
Figure 2: Scan depicting a brain tumor. (Source: Flickr, Michelle Monje).
While there are multiple causes of brain cancer, including genetics and lifestyle choices, the gut microbiome (also affected by genetics and lifestyle choices) may initiate tumorigenesis through its role in the regulation of inflammation, reactions to therapy, and other aspects of the immune response (Mehrian-Shai et al., 2019). Though hypothesized, the gut microbiome’s effect on tumorigenesis was confirmed with a study of Drosophila flies that revealed gut microbiota composition can contribute to the onset of tumors outside the digestive system, including in the brain (Jacqueline et al., 2017). The results of this study demonstrated that non-cancerous larvae had a higher abundance of the bacterial families Bacillaceae and Streptococcus, while cancerous larvae had a higher prevalence of the family Leuconostococaceae within the gut microbiome. However, since this study was the first to suggest that gut microbiome composition can contribute to the onset of cancers outside the digestive system, more research must be conducted to strengthen the link and further prove the association.
Other Cancers
Research has shown that dysbiosis in the gut microbiota can induce carcinogenesis locally as well as systematically, indicating that gut dysbiosis could also influence the development of extraintestinal cancers (Rea et al., 2019; Zitvogel et al., 2015). To this end, certain intestinal ‘microbiota profiles’ (a picture of the different bacterial species that make up the microbiome) have been known to promote hepatocellular carcinoma (HCC), the most common type of liver cancer in adults (American Cancer Society, 2019; Dapito et al., 2012). A 2012 study showed that intestinal gut-microbiota and toll-like receptors or TLRs (membrane proteins that play an important role in the immune system) were not necessarily required for the initiation of HCC development, but both promoted development (Dapito et al., 2012). Furthermore, HCC was reduced when the gut was sterilized at late stages of tumor development, suggesting that the gut-microbiota and certain types of TLRs represent possible therapeutic targets for HCC treatment.
Clinical studies have shown that breast cancer, the most common cancer found in adult women, is also closely associated with gut dysbiosis (Zhang et al., 2019). Genetic and hereditary factors are only responsible for 5-10% of known breast cancer cases, suggesting that lifestyle choices and non-hereditary factors also play a crucial role (Bray et al., 2018; Hashemia et al., 2014). There is also a strong correlation between obesity and the development of breast cancer, and studies have shown that there are close connections between gut dysbiosis and obesity. These studies have shown that a microbiota transplant from an obese patient can induce obesity in a healthy individual (Sung et al., 2018, Leo et al., 2018). Furthermore, the gut microbiomes of breast cancer patients are characterized by decreased overall diversity and increased levels of certain types of bacteria such as Clostridiales as compared with controls, possibly also contributing to disease pathophysiology (Goedert et al., 2015). In addition to HCC and breast cancers, many studies have been conducted to show that gut dysbiosis can be observed in patients with other extraintestinal cancers such as pancreatic, liver, pulmonary, and prostate cancer (Zhang et.al, 2019).
Microbiome of the local tumor microenvironment
In addition to the gut microbiome and the MGBA’s potential impact on cancer development, recent scientific work suggests that the local tissue microbiome may impact cancer development as well. In an effort to investigate this connection, a team of researchers documented the different bacterial species present in more than 1500 tumors and identified whether the bacteria were localized to both cancer and immune cells, finding that tissue microbiome composition varied by cancer type (Nejman et al., 2020). For example, the microbiome of breast cancer was characterized as richer and more diverse than that of other types of cancer with an average of 16.5 bacterial species, while other tumors had less than 9 species. The microbiome of breast tumor samples also had more numerous and diverse species compared to samples from healthy patients, and samples of tissue adjacent to breast tumors showed an intermediate level of bacterial load and diversity. Interestingly, the tumor samples of lung and ovarian tumors did not show these higher levels of bacteria compared to their tumor-adjacent normal samples. Furthermore, it was seen that tumors of the same type from different patients tended to have microbiomes that resembled each other (Nejman et al., 2020).
The same study revealed that specific bacterial metabolic function was associated with the histological features of certain tumors. For example, arsenate detoxification and mycothiol biosynthesis were found to be significantly enriched pathways in bacteria within estrogen receptor-positive (ER+) breast cancers (Nejman et al., 2020). Significantly, arsenic is a breast cancer carcinogen (cancer-causing agent) known to induce expression of estrogen-receptors. Furthermore, mycothiol is found in certain bacteria that help detoxify reactive oxygen species. Researchers hypothesized that bacteria with the ability to synthesize mycothiol are better suited to survive in ER+ tumor environments, which are known to be places of increased oxidative stress (Nejman et al., 2020; Hecht et al., 2016).
Interestingly, different types of tumors are also known to possess varying proportions of bacterial DNA. For example, while more than 60% of breast, pancreatic, and bone tumors contained bacterial DNA, only 14.3% of melanoma tumors, a type of skin cancer, had traces of bacterial DNA (Nejman et al., 2020). This data suggests a link and possibly important relationship between the local tissue microbiome and cancer development, further requiring additional study of the microbiome (both gut and tissue) when studying cancer pathogenesis.
The MGBA and Cancer Treatment
In addition to considering the gut microbiome and MGBA in the context of cancer research, it may also be worth examining the interplay between cancer treatment and the MGBA. It is known that MGBA status can be affected by certain cancer treatments. For instance, it has been proven that microbial diversity decreases significantly as a result of chemotherapy and immunotherapy, as bacterial species L. rhamnosus and L. acidophilus (associated with anti-inflammatory pathways) are seen in lower numbers, thereby giving way to intestinal inflammation and permeability (Song & Bai, 2021). Traditional cancer treatments are also known to induce damage to MGBA-related pathways in the form of neuroinflammation, altered CNS function, and peripheral inflammation (Song & Bai, 2021). Studies have also established a link between chemotherapy-induced dysbiosis and intestinal inflammation, as exposure to chemotherapy can prompt chemotherapy-induced cognitive impairment (CICI) and can cause worsening intestinal inflammation and ulceration (Bajic et al. 2018).
As researchers deepen their understanding of the pathways of the MGBA, there may come the opportunity for breakthrough therapies for the peripheral nervous system (PNS) that can vastly enhance the outcomes of treatments in cancer patients (Song & Bai 2021). Currently, only preclinical work is being done in this area, using rodent models to refine understanding of how physiological regions and processes under the influence of the MGBA such as the vagus nerve, blood brain barrier, and neuroinflammation impact and are impacted by cancer treatments (Song & Bai, 2021).
One notable area of MGBA-related inquiry in cancer research is the use of probiotics for cancer treatment. Probiotics have been shown to help conserve the intestinal barrier, reduce reactive oxygen species, and replace pathogenic bacteria (Song & Bai, 2021). Studies of probiotic use in cancer patients have led to the use of Lactobacillus probiotics to offset diarrhea related to chemotherapy or radiation in patients with pelvic malignancies (Song & Bai, 2021). Moreover, one study illustrated that L. acidophilus and L. rhamnosus treatments may even reduce symptoms such as fatigue, anxiety, and depression in patients who survived colorectal cancer and received chemotherapy (Song & Bai, 2021). Needless to say, there are limitations to such probiotic clinical trials, as they have small sample sizes and a diversity of dosages and probiotic strains that are tested, which can naturally heighten difficulty in making important connections regarding their efficacy (Song & Bai, 2021). Despite this, the role of MGBA-related cancer treatments is being widely explored and many trials are underway.
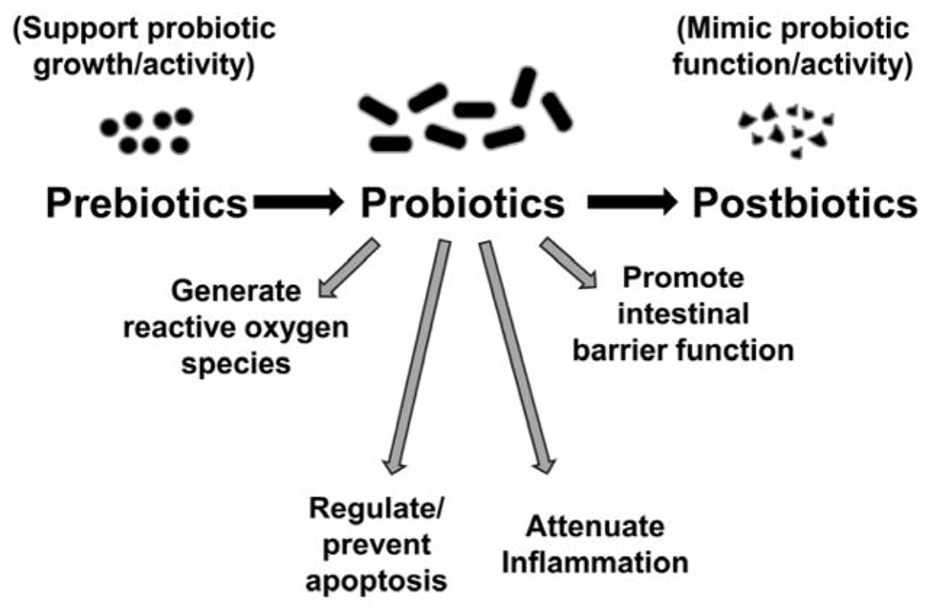
Figure 3: Probiotic therapeutic benefits include anti-inflammatory effects and decreased intestinal permeability, which can counter the dysbiotic-inflammatory pathway of colorectal cancer pathogenesis. (Source: Wikimedia Commons, Ravi Mangal Patel and Patricia Wei Denning).
Conclusion
With recent research revealing the many ways in which the MGBA plays an integral role in cancer pathogenesis, a groundbreaking paradigm shift may be underway with promise for great innovation in the field of oncology. As discussed above, evidence suggests that the MGBA is implicated in not only local cancers such as CRC and brain cancer, but it may also play a role in non-local cancers such as HCC and breast cancer (Rawla et al., 2019; Mehrian-Shai et al., 2019; Dapito et al., 2012; Zhang et al., 2019). Furthermore, recent efforts demonstrate the relevance of the local tumor microbiome to cancer type and development, hinting at the significance of the microbiome as a whole in cancer pathogenesis (Nejman et al., 2020). With this new knowledge, scientists may be able to develop alternative, MGBA-related cancer treatments (like probiotics) that can foreseeably eliminate issues such as toxicity and CICI (Bajic et al., 2018).
References
- Ahmed, Monjur. (2020). Colon Cancer: A Clinician’s Perspective in 2019 | Ahmed | Gastroenterology Research. https://www.gastrores.org/index.php/Gastrores/article/view/1239
- American Cancer Society. (2019, April 1). What Is Liver Cancer? https://www.cancer.org/cancer/liver-cancer/about/what-is-liver-cancer.html
- American College of Surgeons. (2017). Brain Tumor: Grades and Prognostic Factors | Cancer.Net. https://www.cancer.net/cancer-types/brain-tumor/grades-and-prognostic-factors
- Bajic, Juliana E, Johnston, Ian N, Howarth, Gordon S, & Hutchinson, Mark R. (2018, May 22). Frontiers | From the Bottom-Up: Chemotherapy and Gut-Brain Axis Dysregulation | Behavioral Neuroscience. https://www.frontiersin.org/articles/10.3389/fnbeh.2018.00104/full
- Bray, Freddie, Ferlay, Jacques, Soerjomataram, Isabelle, Siegel, Rebecca L, Torre, Lindsey A, & Jemal, Ahmedin. (2018, November). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries—PubMed. https://pubmed.ncbi.nlm.nih.gov/30207593/
- Colucci, Philomena M, Yale, Steven H, & Rall, Christopher J. (2003, July). Colorectal polyps—PubMed. https://pubmed.ncbi.nlm.nih.gov/15931318/
- Cryan et al. (2019, August 28). The Microbiota-Gut-Brain Axis | Physiological Reviews. https://journals.physiology.org/doi/full/10.1152/physrev.00018.2018
- Dapito, D. H., Mencin, A., Gwak, G.-Y., Pradere, J.-P., Jang, M.-K., Mederacke, I., Caviglia, J. M., Khiabanian, H., Adeyemi, A., Bataller, R., Lefkowitch, J. H., Bower, M., Friedman, R., Sartor, R. B., Rabadan, R., & Schwabe, R. F. (2012). Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell, 21(4), 504–516. https://doi.org/10.1016/j.ccr.2012.02.007
- Di, Yang-Zi, Han, Bo-Sheng, Di, Jun-Mao, Liu, Wei-Yan, & Tang, Qiang. (2019, July 6). Role of the brain-gut axis in gastrointestinal cancer—PubMed. https://pubmed.ncbi.nlm.nih.gov/31367615/
- Ge, Yanshan, Wang, Xinhui, Guo, Yali, Yan, Junting, Abuduwaili, Aliya, Aximujiang, Kasimujiang, Yan, Jie, & Wu, Minghua. (2021, January 25). Gut microbiota influence tumor development and Alter interactions with the human immune system | Journal of Experimental & Clinical Cancer Research | Full Text. https://jeccr.biomedcentral.com/articles/10.1186/s13046-021-01845-6
- Goedert, James J, Jones, Gieira, Hua, Xing, Yu, Guoqin, Flores, Roberto, Falk, Roni T, Gail, Mitchell H, Shi, Jianxin, Ravel, Jacques, & Feigelson, Heather Spencer. (2015, June 1). Investigation of the Association Between the Fecal Microbiota and Breast Cancer in Postmenopausal Women: A Population-Based Case-Control Pilot Study. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4554191/
- Hashemi, Seyed Hesam Bani, Karimi, Samieh, & Mahboobi, Hamidreza. (2014, July 1). Lifestyle changes for prevention of breast cancer. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4324293/
- Hasstedt, N. (2019). The Role of the Gut Brain Axis and Microbiome in Colorectal Cancer, Alzheimer’s Disease, and Crohn’s Disease. Iowa State University Capstones, Theses, and Dissertation, 24.
- Hasstedt—The Role of the Gut Brain Axis and Microbiome in C.pdf. (n.d.). Retrieved June 25, 2021, from https://lib.dr.iastate.edu/cgi/viewcontent.cgi?article=1193&context=creativecomponents
- Hecht, Fabio, Pessoa, Carolina F, Gentile, Luciana B, Rosenthal, Doris, Carvalho, Denise P, & Fortunato, Rodrigo S. (2016, January 27). The role of oxidative stress on breast cancer development and therapy | SpringerLink. https://link.springer.com/article/10.1007/s13277-016-4873-9
- Jacqueline, C., Brazier, L., Faugère, D., Renaud, F., Thomas, F., & Roche, B. (2017). Can intestinal microbiota be associated with non-intestinal cancers? Scientific Reports, 7(1), 12722. https://doi.org/10.1038/s41598-017-11644-9
- Lawrence, T. (2009). The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harbor Perspectives in Biology, 1(6), a001651. https://doi.org/10.1101/cshperspect.a001651
- Marmol, Ines, Sanchez-de-Diego, Cristina, Dieste, Alberto Pradilla, Cerrada, Elena, & Yoldi, Maria Jesus Rodriguez. (2017, January 19). IJMS | Free Full-Text | Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. https://www.mdpi.com/1422-0067/18/1/197
- Martinez Leo, Edwin E, Martin Ortega, Armando M, & Segura Campos, Maira R. (2018). Gut Microbiota Alterations in People With Obesity and Effect of Probiotics Treatment—ScienceDirect. https://www.sciencedirect.com/science/article/pii/B9780128114407000053
- Mayo Clinic Staff. (n.d.). Brain tumor—Symptoms and causes—Mayo Clinic. Retrieved June 25, 2021, from https://www.mayoclinic.org/diseases-conditions/brain-tumor/symptoms-causes/syc-20350084
- Mehrian-Shai, Ruty, Reichardt, Juergen KV, Harris, Curtis C, & Toren, Amos. (2019, March 15). The Gut–Brain Axis, Paving the Way to Brain Cancer: Trends in Cancer. https://www.cell.com/trends/cancer/fulltext/S2405-8033(19)30026-3?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS2405803319300263%3Fshowall%3Dtrue
- Murthy, Vivek H, Krumholz, Harlan M, & Gross, Cary P. (2004, June 9). Participation in Cancer Clinical Trials: Race-, Sex-, and Age-Based Disparities | Lung Cancer | JAMA | JAMA Network. https://jamanetwork.com/journals/jama/fullarticle/198896
- National Cancer Institute. (2013, May 9). Tumor Grade Fact Sheet (nciglobal,ncienterprise) [CgvArticle]. https://www.cancer.gov/about-cancer/diagnosis-staging/prognosis/tumor-grade-fact-sheet
- Nejman et al. (2020, May 29). The human tumor microbiome is composed of tumor type–specific intracellular bacteria | Science. https://science.sciencemag.org/content/368/6494/973
- Rawla, Prashanth, Sunkara, Tagore, & Barsouk, Adam. (2019, February). Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. https://www.termedia.pl/Epidemiology-of-colorectal-cancer-incidence-mortality-survival-and-risk-factors,41,34580,0,1.html
- Rea, Domenica, Coppola, Giovanni, Palma, Giuseppe, Barbieri, Antonio, Luciano, Antonio, Del Prete, Paola, Rossetti, Sabrina, Berretta, Massimiliano, Facchini, Gaetano, Perdona, Sisto, Turco, Maria Caterina, & Arra, Claudio. (2018, April 3). Microbiota effects on cancer: From risks to therapies. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5915165/
- Seruga et al. (2014). Barriers and Challenges to Global Clinical Cancer Research—Seruga—2014—The Oncologist—Wiley Online Library. https://theoncologist.onlinelibrary.wiley.com/doi/full/10.1634/theoncologist.2013-0290
- Song, B. C., & Bai, J. (2021). Microbiome-gut-brain axis in cancer treatment-related psychoneurological toxicities and symptoms: A systematic review. Supportive Care in Cancer, 29(2), 605–617. https://doi.org/10.1007/s00520-020-05739-9
- Sung, H., Siegel, R. L., Torre, L. A., Pearson-Stuttard, J., Islami, F., Fedewa, S. A., Goding Sauer, A., Shuval, K., Gapstur, S. M., Jacobs, E. J., Giovannucci, E. L., & Jemal, A. (2019). Global patterns in excess body weight and the associated cancer burden. CA: A Cancer Journal for Clinicians, 69(2), 88–112. https://doi.org/10.3322/caac.21499
- World Health Organization. (2021, March 3). Cancer. https://www.who.int/news-room/fact-sheets/detail/cancer
- Zhang, Ziying, Tang, Haosheng, Chen, Peng, Xie, Hui, & Tao, Yongguang. (2019, October 12). Demystifying the manipulation of host immunity, metabolism, and extraintestinal tumors by the gut microbiome. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6799818/
- Zitvogel, L., Galluzzi, L., Viaud, S., Vétizou, M., Daillère, R., Merad, M., & Kroemer, G. (2015). Cancer and the gut microbiota: An unexpected link. Science Translational Medicine, 7(271), 271ps1. https://doi.org/10.1126/scitranslmed.3010473
Related Posts
The Pathway to Sonogenetics: Understanding the Mechanical Nature of the Ultrasound Stimulation Mechanism in C. Elegans
Figure 1: C. elegans in Agar under Microscope Magnification Source:...
Read MoreThe Mediterranean Diet Could Help Treat and Prevent Adolescent Depression
Source: Mediterranean Diet Pyramid (Flikr Public Domain, Zaid Alasad) The...
Read MoreThe “Old Friends” Hypothesis and the COVID-19 Pandemic
Authors: Sakeena Badrane1, Vivek Babu2, Sanah Handu1 1University of Pittsburgh,...
Read More3D Bioprinting: An Innovative Approach to Promote Cartilage Regeneration
Source: Laboratoires Servier, (CC BY-SA 3.0) Many of us cannot...
Read MoreFighting Hospital Hardships: An Intervention for Reducing Stress in Hospital Workers
Figure 1: This figure describes some of the symptoms of...
Read MoreAlleviating Vaccine Hesitancy: The Path to a Safer Future
The human mind is constantly working on the fly, adapting...
Read MoreJillian Troth, Roxanna Attar, Julia Gainski, Vincent Lai, Grace Lee, Callie Moody, Megan Nelson