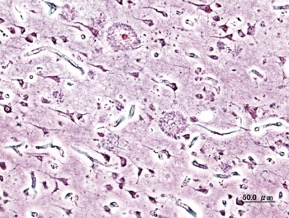
Figure 1: Extracellular deposits of amyloid-beta proteins as seen in Alzheimer’s Disease patients
Source: Wikimedia Commons
As of June 2020, Alzheimer’s Disease (AD) was the sixth most common cause of death in the United States, as well as the leading cause for the onset of dementia. Largely due to continuously increasing life expectancies, it is currently estimated that the incidence of AD and dementia could rise globally in the coming decades (Turner et al., 2020). Fortunately, it is not set in stone that this prediction will come true. In fact, data out of varying regions of the United Kingdom have suggested that the incidence of AD in those aged 65 years and older is actually decreasing. Researchers believe this fact is likely thanks to those populations’ limited incidence of health factors that are known to contribute to high risk for AD, including type 2 diabetes, smoking, and obesity (Turner et al., 2020). It is thus important to recognize that awareness and prevention of these and other risk factors could have implications for the future prevalence of AD.
Of course, attention to certain risk factors will not stop Alzheimer’s Disease altogether. As far as AD cases that will inevitably happen, due to strong genetic predisposition, there are significant and ongoing developments concerning progress toward earlier and more accurate diagnoses. Research in this field could result in new and efficient methods for early detection of AD, leading to a variety of benefits. First and foremost, the patients themselves will benefit greatly from these tools; not only will they still have the mental capacity to make their preferences known regarding care, but they will also be able to maintain an increased quality of life for a prolonged period of time. Caretakers will also benefit from these circumstances, with more time both to emotionally process the unfolding events and to make necessary adjustments to plans and lifestyles. Even the economy could benefit from early diagnostic tools, with some models suggesting that the caretakers of AD patients would incur less costs in the long run as a result of the disease being addressed beginning in its earlier stages (Rasmussen & Langerman, 2019).
Much of the progress in research regarding diagnostic techniques has involved the hallmark contributors to Alzheimer’s Disease, including amyloid beta-derived diffusible ligands (ADDL’s) and abnormally hyperphosphorylated tau proteins (Yang et al., 2019). In 2004, scientists were successfully able to use nanoscale biosensor technology to “monitor the interaction between the antigen, ADDL’s, and specific ADDL antibodies” thereby allowing for the “determination of ADDL concentration.” This was a very significant step in the path to AD diagnosis, as knowledge of the interactions between these biological species at their native concentrations would allow scientists to study “drug interactions with target species” (Haes et al., 2005). As time passes, research on these hallmark pathogens continues to evolve. Within the past few years, scientists have been working to develop and improve near-infrared fluorescent (NIRF) probes for the imaging of amyloid-beta aggregates and hyperphosphorylated tau protein (Yang et al., 2019). Continued work in this area (better understanding the pathological hallmarks and developing NIRF probes) could be helpful for the diagnosis of AD.
In particular, one very interesting area of research in the field has been the prospective use of A Disintegrin And Metalloprotease 10 (ADAM10) as “a blood biomarker for the early diagnosis of AD” (de Oliveira et al., 2020). Essentially, ADAM10 cleaves amyloid-beta precursor protein; this cleavage results in the formation of amyloid-beta peptides, which as previously mentioned is a pathological hallmark of Alzheimer’s (Manzine et al., 2019). Consequently, this information would suggest that higher concentrations of ADAM10 correlate with more severe disease, or the general presence of disease as opposed to none at all. In 2020, this was shown to be true when ADAM10 detection using a disposable microfluidic platform revealed that the mean concentration of ADAM10 was 1419 pg/mL for healthy controls, 1935 pg/mL for patients with mild AD, and 2244 pg/mL for those with the most severe AD (de Oliveira et al., 2020).
Together, this indicates that successful detection of ADAM10 concentrations could be extremely useful in the early diagnosis of AD. In fact, there is diagnostic potential for the very disposable microfluidic platform technology that was used to compare groups in the aforementioned study. One benefit of this method is that it is both low-cost and simple relative to many other possibilities in the field. Also, the technology has an extremely low limit of detection, specifically 0.35 fg/mL, or “80,000 times lower than the value provided by the commercial enzyme-linked immunosorbent assay (ELISA) kit manufacturer.” The implication of this fact would be that the technology offers an extraordinarily minimally-invasive technique for patients, since such “low volumes of sample are required for the analysis.” This platform was also demonstrated to yield a high accuracy in differentiation between individuals (de Oliveira et al., 2020).
Research over both the past couple years as well as the past couple decades seems to suggest that there is promise in continuing to look into possibilities regarding the detection of pathological hallmarks of Alzheimer’s Disease. Given that ADAM10 is directly related to the aggregation of amyloid-beta peptides, there is also the potential that further study of the mechanisms of hallmark pathogens could give rise to discovery of and experimentation with other Alzheimer’s-associated molecules. This would be helpful for early diagnosis and provide abetter understanding of the disease in general (Manzine et al., 2019). These methods, in combination with the improvement of existing technologies, will lead to a future where a vast majority of AD patients will experience an increased quality of life thanks to the early diagnosis of their disease.
References
De Oliveira, Tassia R, et al. (2020). “Early Diagnosis of Alzheimers Disease in Blood Using a Disposable Electrochemical Microfluidic Platform.” American Chemical Society. pp. 1010–1019., doi:10.1021/acssensors.9b02463.s001.
Haes, Amanda J., et al. (2005). “Detection of a Biomarker for Alzheimer’s Disease from Synthetic and Clinical Samples Using a Nanoscale Optical Biosensor.” Journal of the American Chemical Society 127, no. 7, pp. 2264–2271., doi:10.1021/ja044087q.
Manzine, Patricia Regina, et al. (2019) “ADAM10 In Alzheimer’s Disease: Pharmacological Modulation by Natural Compounds and Its Role as a Peripheral Marker.” Biomedicine & Pharmocotherapy. reader.elsevier.com/reader/sd/pii/S075333221837032X?token=5E731F8C93A2CBB4FC2059E9A294667CF4EB9CAC639D9EA062BCC51D0B826A0983F86565B0F30F4327647B54B133C680.
Rasmussen, Jill, and Haya Langerman. (2019). “Alzheimer’s Disease – Why We Need Early Diagnosis.” Degenerative Neurological and Neuromuscular Disease, www.ncbi.nlm.nih.gov/pmc/articles/PMC6935598/.
Turner, R Scott, et al. (2020). “Potential New Approaches for Diagnosis of Alzheimer’s Disease and Related Dementias.” Frontiers in Neurology, Frontiers Media S.A www.ncbi.nlm.nih.gov/pmc/articles/PMC7290039/.
Yang, Jian, et al. (2019). “Development of Near-Infrared Fluorescent Probes for Use in Alzheimer’s Disease Diagnosis.” Bioconjugate Chemistry.
Related Posts
Health Literacy in the United States
This publication is in proud partnership with Project UNITY’s Catalyst...
Read MoreThe Gut May Be The Door to Effective Depression Treatment
First Author: Jillian Troth1 Co-Authors [Alphabetical Order]: Roxanna Attar2, Caroline...
Read MoreA Spoonful of (Modified) Sugar as an Antiviral Medicine
Figure 1: A negative stain transmission electron micrograph of Influenza...
Read MoreMatthew Lutchko



Comments are closed.