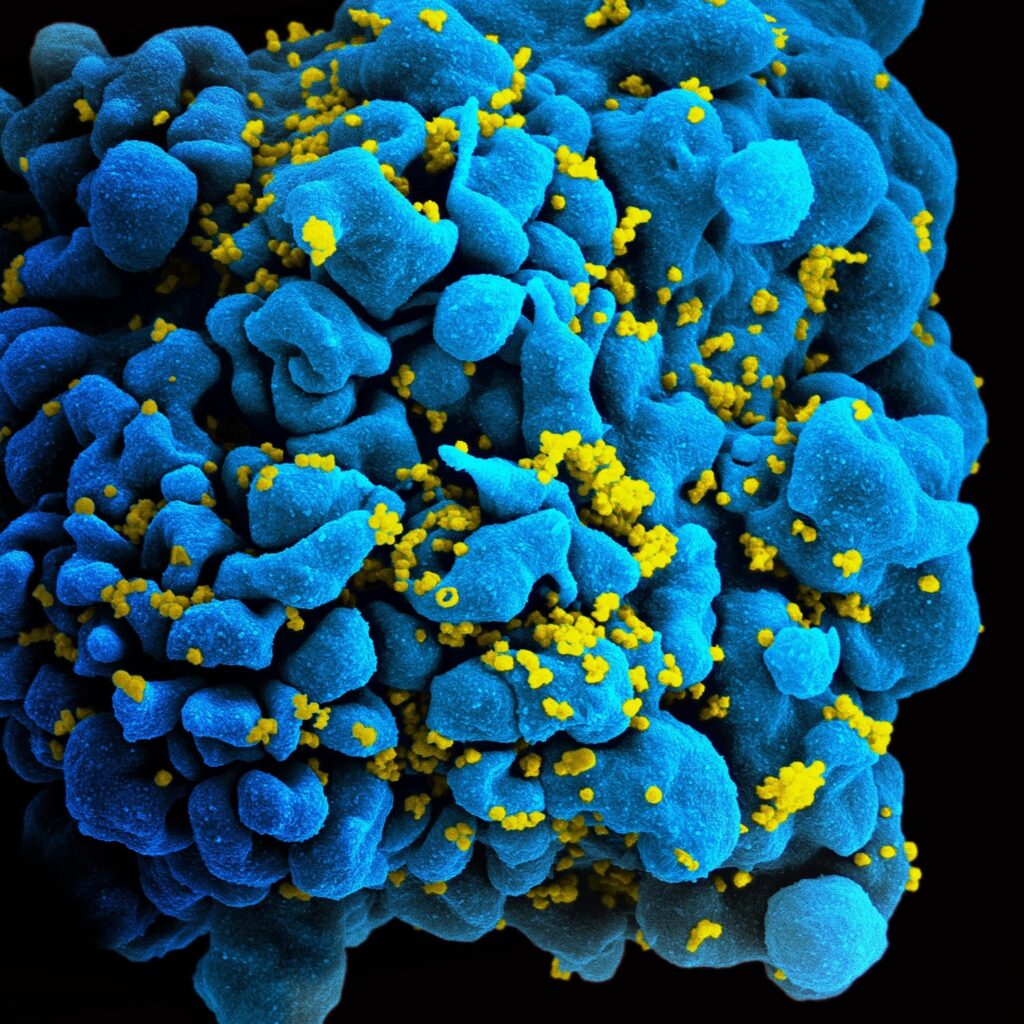
Figure 1: SEM image of the human immunodeficiency virus (HIV) budding from a human T cell.
Since their clinical discovery in 1981, the human immunodeficiency virus (HIV) has been one of the most deadly and difficult to treat pathogens. The trouble in finding a cure for HIV is largely due to the pathogen’s ability to lay dormant in immune cells, shielding itself from detection by the body. However, researchers at The Wistar Institute Vaccine and Immunotherapy Center may have identified the key to finding these hiding places for HIV-1 Group M (the most widespread strain and that which is responsible for the AIDS epidemic), potentially a major step in developing more effective treatments. For the purposes of this article, we will just be discussing
HIV-1 is highly transmittable via bodily fluids and causes acquired immunodeficiency syndrome (AIDS), a progressive failure of the immune system that leaves the body susceptible to opportunistic infections and cancers. Current HIV treatment can slow the progression of AIDS and curb complications, but no cure for HIV yet exists.
HIV’s virulence is primarily due to its mechanism of attack. It targets the helper T-cells, a group of immune cells necessary for activating the majority of the remaining immune system. Without the helper T-cell signaling, the immune system can only mount a crippled response, which often leads to a quicker development and severity of infections in the body.1
Viral research for HIV has been stagnant, confounded by the pathogen’s ability to resist standard viral treatment by hiding within the cell. HIV is a retrovirus, meaning it reproduces by inserting its RNA into the host cell’s DNA. The host cell then reads this RNA as its own, thereby reproducing viral RNA. In most cases, the infected cell will reproduce for one to two days and then eventually be killed. In a small percentage of cells, however, the virus does not actively reproduce but lies latent and hidden in the infected cell’s DNA.
Standard antiretroviral therapy (ART) has proven effective in preventing the infection of new T-cells but cannot treat the virus already hidden in the hosts.3 When treatment is discontinued, the virus will reappear from the infected immune cell’s genomes and the disease progress will resume.2 As such, HIV proves difficult to cure completely without targeting these hidden viral reservoirs.
Identifying key characteristics of latently infected cells, cells that house the hidden virus, is a necessary precursor for HIV treatment. One promising avenue of identification is the unique surface sugar configuration of the cell, known as the glycome. Every cell has this unique configuration of proteins and sugars on the cell surface, and recognizing this array of surface molecules is central to the immune defense. However, each class of cell will display the same surface glycome; in the case of HIV it was believed that healthy T-cells appeared the same as diseased cells.
However, when examining these surface signatures, the team at the Wistar Institute identified a unique glycome component of actively infected cells that they predict to be a byproduct of HIV transcription. The team used fluorescent proteins to separate cells into HIV-infected transcriptionally active, HIV-infected transcriptionally inactive (dormant), and uninfected cells. They then used a lectin microarray to examine multiple glycome structures on each of these cells.1
Researchers found slight differences in the surface glycomes of HIV-infected cells when compared to healthy cells. The most notable change was the fucosylation, or attachment of a small fucose sugar, of the surface of HIV-infected cells undergoing active transcription.3 Specifically, the team found the fucosylated antigen Sialyl-LewisX (SLeX) present on cells displaying active HIV transcription. This antigen was not found on dormant infected cells, allowing for possible differentiation between actively transcribing and dormant infected cells.3
High SLeX levels are also found in some metastasizing cancer cells, allowing them to spread due to enriched molecular pathways involved in moving blood and tissue.3 The researchers believe these enhanced levels of movements might help explain the persistence of HIV in tissue.
The role of fucosylation of surface glycomes requires further research to identify its exact contribution to persistence as well as possible mechanisms for targeting these HIV reservoirs in blood and tissue. However, identification of these characteristics is a promising advancement in improved HIV treatment.
References
- Bruner K.M., Hosmane N. N., Siliciano R. F. (2015). Towards an HIV-1 cure: measuring the latent reservoir. Trends in Microbiology. 23(4), 192-203. https://doi.org/10.1016/j.tim.2015.01.013
- Reardon, S. (2018) Virus detectives test whole-body scans in search of HIV’s hiding places. Nature, 562, 472-473. https://doi.org/10.1038/d41586-018-07115-4
- Colomb et al., (2020). Sialyl-Lewis Glycoantigen is Enriched on Cells with Persistent HIV Transcription during Therapy. Cells Report, 32(5) 107991. https://doi.org/10.1016/j.celrep.2020.107991
Related Posts
Exploring Possibilities for the Early Diagnosis of Alzheimer’s Disease
Figure 1: Extracellular deposits of amyloid-beta proteins as seen in...
Read MoreThe Biochemistry and Broad Utility of Pfizer’s New COVID-19 Drug: Paxlovid
A photo of a Pfizer facility in Italy. (Image Source:...
Read MoreRethinking ‘Junk’ DNA: The Vital Role DNA Repeats Play in Cancer and Aging
Figure: The image above, created by the National Institutes of...
Read MoreSean McOsker