Figure: The image above, created by the National Institutes of Health, helps demonstrate how important a role junk DNA can play. The picture displays a stem cell culture, within which green dots represent viral envelope proteins resulting from the transcription and subsequent translation of junk DNA. When these proteins interact with certain immune cell proteins, this can prevent the stem cells from developing into neurons. However, this outcome can be avoided if the relevant junk DNA are rendered inactive, illustrating how these so-called ‘junk’ sequences are far from non-impactful. (Source: Wikimedia Commons, NIH)
When the Human Genome Project concluded, scientists were left with roughly 85% of the full human DNA sequence and many revelations. One revelation was the identification of so-called “junk DNA” — repetitive DNA sequences that do not play a role in protein synthesis. It is estimated that nearly half the human genome consists of this junk DNA (Van Dongen, 2021). Despite their repetitive nature, junk DNA sequences can vary between individuals, contributing to genetic diversity (Xu et al., 2021). Initially, researchers believed that these sequences lived up to their name, serving no essential function. Studies in recent years, however, have called into question whether junk DNA can truly be dismissed as mere “junk.”
It is now believed that junk DNA sequences are key to the regulation of gene expression and play a major role in disease susceptibility (Xu et al., 2021). The newfound appreciation for junk DNA in the scientific community is partly the result of a general expansion in focus beyond exclusively those DNA sequences that contribute to protein synthesis. Junk DNA often codes for RNA sequences that will never be translated to proteins, and these RNA sequences can play valuable roles in cell function. The Encyclopedia of DNA Elements found that 80% of human DNA serves some biochemical function, meaning a fair portion of junk DNA must have purpose (Pennisi, 2012). One of the latest junk DNA sequences to be studied in detail is the variable number tandem repeat sequence VNTR2-1, which Xu et al. (2021) now believe regulates telomerase gene activity.
When cells age, they stop dividing and replicating. Telomeres play a key role in this aging process. Telomeres are protective caps on the end of DNA strands that get progressively shorter with each duplication. When a telomere becomes too short in an aging cell, duplication is no longer possible, and the cell will ultimately die (Van Dongen, 2021). This is how most adult cells function. Enter telomerase reverse transcriptase; this enzyme facilitates telomere length homeostasis, meaning telomere length is maintained across cell divisions. The hTERT gene codes for telomerase reverse transcriptase and is most active in tissues with abundant stem cells and in cancer cells (Xu et al., 2021). Xu et al. (2021) found that the VNTR2-1 sequence serves as a sort of enhancer, meaning it helps activate the transcription of its associated gene (hTERT) when bound by transcription factor proteins. Excitingly, they also found that when this activation process is disrupted by the deletion of the VNTR2-1 sequence from cancer cells, telomeres begin to shorten with each cell division, and tumor growth is halted. This provides vital insight into factors influencing cancer beyond oncogenes (mutated forms of genes involved in healthy cell growth that can cause cancer cells to grow) and tumor suppressor genes (genes that ordinarily regulate cell division but, when dysfunctional, can allow cancerous growth). Oncogenes and tumor suppressor genes currently fail to fully explain cancer risk for patients (Van Dongen, 2021).
It is also interesting to note that the number of DNA repeats within the VNTR2-1 sequence varies between individuals. Xu et al. (2021) studied populations with VNTR2-1 sequences ranging between 53 and 160 repeats and found that a longer sequence of repeats is correlated with a higher level of activity of the hTERT gene. The study found distinctions in VNTR2-1 length between demographic groups, noting that African American subjects had the shortest VNTR2-1 sequences. Having a short VNTR2-1 sequence is a double-edged sword, since it could mean a reduced risk of developing cancer but might also indicate more rapid cellular aging (Van Dongen, 2021).
Telomerase activity is certainly a factor scientists will continue to focus on in the fight against cancer and in the effort to combat the biological consequences of aging. One study on telomerase-deficient mice has already determined that telomerase reactivation can lead to the resumption of cell proliferation, a reduction in DNA damage, and the extension of telomeres. Furthermore, telomerase reactivation eliminated degenerative organ phenotypes and appeared to reverse neurodegeneration (Jaskelioff et al., 2011). Clearly, a knowledge of telomerase function and regulation is essential to the medical community. Hopefully future studies will be able to harness the research community’s newfound VNTR2-1 knowledge to develop novel cancer and anti-aging treatments.
References
Jaskelioff, M., Muller, F. L., Paik, J.-H., Thomas, E., Jiang, S., Adams, A. C., Sahin, E., Kost-Alimova, M., Protopopov, A., Cadiñanos, J., Horner, J. W., Maratos-Flier, E., & DePinho, R. A. (2011). Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature, 469(7328), 102–106. http://dx.doi.org.dartmouth.idm.oclc.org/10.1038/nature09603
Pennisi, E. (2012). ENCODE project writes eulogy for junk DNA. Science, 337(6099), 1159–1161. https://doi.org/10.1126/science.337.6099.1159
Van Dongen, J. (2021, July 23). Potential role of ‘junk DNA’ sequence in aging, cancer. ScienceDaily. https://www.sciencedaily.com/releases/2021/07/210723105258.htm
Xu, T., Cheng, D., Zhao, Y., Zhang, J., Zhu, X., Zhang, F., Chen, G., Wang, Y., Yan, X., Robertson, G. P., Gaddameedhi, S., Lazarus, P., Wang, S., & Zhu, J. (2021). Polymorphic tandem DNA repeats activate the human telomerase reverse transcriptase gene. Proceedings of the National Academy of Sciences, 118(26). https://doi.org/10.1073/pnas.2019043118
Related Posts
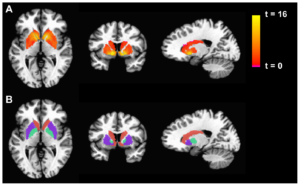
Recent Study Conducted by Duke University Researchers Reveals Critical Limitation of fMRI Measurements
Figure 1: fMRI is used to display differences in basal...
Read MoreThe Cognitive Aspects Behind Computer Code Comprehension
Figure 1: A section of a python code program. Programming...
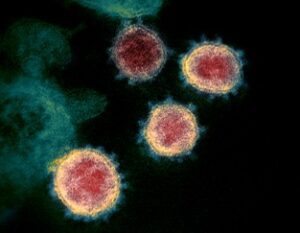
Read MoreCOVID-19 May Inflict Lasting Damage on the Central Nervous System
Figure 1: Transmission electron microscope image of the SARS-CoV-2 virus....
Read MoreWhy Future A.I Robots Should Be Created With Immutable Code Limiting Singularity
Figure: Sophia, the first robot citizen in the Global A.I...
Read MoreHow Tele-Mental Health Helps Us During the COVID-19 Pandemic
Figure 1: Telehealth consultation via smartphones can serve as a...
Read MoreCaroline Conway