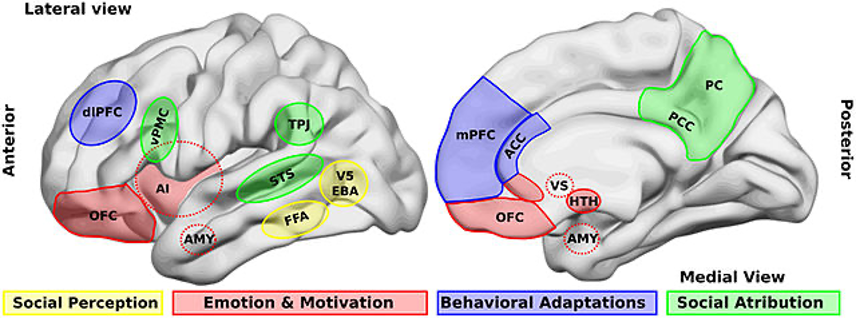
Figure: Illustration of brain areas that are involved in social processing (Source: Wikimedia Commons, Billeke P & Aboitiz F, 2013)
Have you ever had this experience? On a drowsy afternoon at work, you are extremely sleepy. You eat a snack or have a chat with your colleagues and you quickly bounce back. In this scenario, two basic human needs are being met and working their magic on the brain: eating and socializing. Specifically, it seems that eating and socializing re-activate the brain that was earlier “hacked” by sleeping.
The brain is a very busy organ, working to control our thought, emotion, memory, motor movement, and metabolism activities all at once (Gunnell, et al., 2019). Great research effort has been invested to understand the neural foundations of human behaviors. Traditionally, brain partitions have been defined as a way to understand bow brain organization suits cognitive function, with a range of cognitive functions mapped to corresponding anatomical brain regions (Alchihabi et al., 2021). This method of brain division may make us habitually believe that each brain area is only responsible for a specific function or several functions, and there are strict boundaries between them (Sun et al., 2018). For example, many believe that the prefrontal cortex is responsible for executive function, and the feeling and expression of fear is solely the amygdala’s job (Kesler et al., 2011).
Yet scientists have recently argued that this emphasis on regional classification can be misleading in understanding how the brain is structured and work, and may even restrain the development of treatments for brain damage (Jordana, 2021). As a consequence, a new framework for brain research is emerging. It proposes that researchers should look for what kind of “information combination” each region represents, rather than dividing different cortical regions and linking each region to a visual, auditory, somatosensory, or executive function (Shine et al., 2019).
There are many clear biological reasons why this older framework should be updated. For a start, these well-established regions additionally work for other human activities, and the normal work of these abilities may simultaneously activate multiple brain networks (Tanaka & Kazumasa Z., 2021). For example, the lateral hypothalamus (LH) was found to govern the regulation of many behavioral and physiological processes including feeding, arousal, energy balance, stress, reward, motivated behaviors, digestive functions, and blood pressure (Sheth et al., 2017). More Specifically, three different cell populations (i.e., LH MCH, orexin/hypocretin, and GABAergic cell populations) regulate energy homeostasis-related activities, such as feeding and sleep-wake (Arrigoni et al., 2019; Willie et al., 2001). Another example is that impairments in the hippocampus may cause damage to much more than memory (Castaldi et al., 2020; Tanaka & Kazumasa Z., 2021). Contrary to popular belief, the hippocampus does not work exclusively for memory but also other human activities (Zhu et al., 2019).
Moreover, the traditional research framework may generate confusing results. For example, to find in what regions the human brain performs different functions, neuroscientists typically correlate cognitive processes with patterns of brain activity measured by fMRI (Jiang et al., 2020). However, inconsistent findings and mixed results often occur, and studies suggest that this may be because irrelevant muscle twitches and fidgets contaminate the measurement readings (Cohen et al., 2014). Furthermore, when scientists obtain results to prove a hypothesis, it may only reflect characteristics under certain task requirements or experimental contexts. That is, we may know a lot about how the brain operates in a carefully controlled laboratory setting, but not how the brain operates in a real-world setting. Again, the theory that the brain can be split anatomical regions with corresponding functions and boundaries is not completely wrong – it is accurate to an extent – but it oversimplifies the complexity of brain function; the reality that different brain regions have multiple functions, and neurological processes like consolidating memory involve a combination of brain regions.
To address the shortcomings of the old ‘regional’ paradigm of brain function, the new research perspective proposes that it is more desirable to consider the joint functional structures of the brain (Jordana, 2021). In this new perspective, we see brain regions with flexible boundaries and sharing functions. Back to our example at the beginning of this article, stimuli from satisfying basic human needs like eating, socializing, and sleeping activate neural reward systems (Wang et al., 2019). Depriving social needs evokes social cravings, analogous to the way dieting evokes food craving and sleeplessness evokes sleep craving (Parker et al., 2022; Schienle et al., 2022). Therefore, it is assumed that these three human behaviors – eating, sleeping, and socializing – may be related to the same or similar brain networks.
In a recent study, researchers subjected participants to social and eating deprivation for 10 hours (Tomova, L. et al., 2020). After deprivation, they found participants’ midbrain regions (more specifically the dopaminergic midbrain neurons) were selectively activated due to both food cues after fasting and to social cues after isolation (Tomova, L. et al., 2020). Similarly, sleep disturbance significantly impairs central brain mechanisms governing appetitive food desire, involving activity reduction in the frontal cortex and insular cortex and amplification in the amygdala (St-Onge et al., 2012). Restricted sleep likewise affects hormones related to appetite, resulting in increased susceptibility to food stimuli (in other words, people tend to be more hungry when they are tired). One involved mechanism is that of neural reward areas including the putamen, nucleus accumbens, thalamus, insula, and prefrontal cortex in response to food stimuli (St-Onge et al., 2012).
In another study, a propagating, self-reinforcing cycle of social withdrawal and sleep loss was proposed (Ben Simon et al., 2018). In this cycle, lack of sleep triggers a neural and behavioral phenotype of social separation, which leads to a greater reduction in sleep quality. Interestingly, Goldstein-Piekarski et al. (2015) and Simon et al. (2020) also demonstrated that sleep deprivation impairs the social brain, particularly, the viscerosensory brain (anterior insula, anterior cingulate cortex, amygdala), deteriorating the normally reciprocal associations between these central and peripheral emotion-signaling systems.
Shifting our research paradigm of how the brain’s architecture determines function can be uncomfortable and even painstaking, but it has many potential advantages. As for methodological consideration, reduced reliance on artificial manipulation may help deal with the “Reproducibility Crisis” in psychological research. In psychology research, it is not easy to replicate existing findings, even if you’ve obtained the shared data and scripts. Others will inevitably encounter all kinds of problems during the verification process of your work as well. The “Reproducibility Crisis” facing psychological science is widely believed to stem from flaws in theoretical frameworks, methodology, or statistical analyses. Empirical research stemming from subjective intuition or restrained within a particular experimental setting exacerbates this situation. Instead, new research frameworks in this article could reduce reliance on artificial manipulation, and therefore, improve the “Reproducibility Crisis”. Concerning theoretical foundation, instead of studying separate functions with distinct labeled brain regions, brain research that emphasizes comprehensive function and dynamic connectivity allow for a deeper investigation of brain science.
Furthermore, a transition away from experimental procedures that are too sophisticated or condition-restrained to relate to real-life situations would promote advances in mental illness treatment – which is ultimately the chief goal on the minds of most neuroscientists and the broader public. To apply this new research perspective, scientists are now considering whole-brain neural activity and an assortment of behaviors at the same time (Sporns, O., & Betzel, R. F., 2016; Todd et al., 2020). In other words, we study the whole system as its parts interact. For example, to improve the current Internet gaming disorder (IGD) diagnostic criteria proposed in the DSM-5, researchers recorded participants’ resting-state data using fMRI. Based on regional and inter-regional brain features, multivariate pattern analyses of the whole-brain functional connectivity suggest that brain regions involved in the default mode network and executive control network are significantly related to the core criteria for IGD (Dong et al., 2020). Another study also focused on the whole-brain structural brain connectivity but used task-state data (Disselhoff et al., 2020). They used the stop-signal paradigm and the D-KEFS′ Color-Word Interference Test to test inhibition impairments in children. Results found that preterm birth results in long-term alterations of structural connectivity at the network level, and a network of parietal, cerebellar, and subcortical connections is associated with inhibition abilities irrespective of birth status. Taken together, the evaluation of this new framework does not depend on how it explains unresolved questions left, but rather how it generates new problems. This is because the problems brought about by the older research framework inspire the new view. In other words, we advance and progress due to arising problems not to solving old ones. Taken together, the author sees the new brain perspective, not as a replacement to the traditional anatomy approach, but as complementary. Research in this “shared” or “combined” perspective requires further consideration. The current studies overall are limited by the ambiguity of observed correlations (Schmid, G. et al., 2010). For instance, correlations between questionnaire scores and neuroimaging indicators may reveal mechanisms underlying study variables. However, there is no guarantee that the study variable is only related to this part of the brain, and it is difficult to advance the study of causality. Nonetheless, we need to re-consider the brain in light of this new research paradigm of combined functions rather than operating on the assumption that individual brain regions are responsible for distinct functions.
References
Alchihabi, A., Ekmekci, O., Kivilcim, B. B., Newman, S. D., & Yarman Vural, F. T. (2021). Analyzing Complex Problem Solving by Dynamic Brain Networks.Frontiers in Neuroinformatics, http://dx.doi.org/10.3389/fninf.2021.670052
Arrigoni, E., Chee, M. J., & Fuller, P. M. (2019). To eat or to sleep: That is a lateral hypothalamic question. Neuropharmacology, 154, 34-49.
Ben Simon, E., Walker, M.P. Sleep loss causes social withdrawal and loneliness. Nat Commun 9, 3146 (2018). https://doi.org/10.1038/s41467-018-05377-0
Castaldi, E., Lunghi, C., & Morrone, M. C. (2020). Neuroplasticity in adult human visual cortex. Neuroscience and Biobehavioral Reviews, 112, 542-552. http://dx.doi.org/10.1016/j.neubiorev.2020.02.028
Cohen, M. X., & van Gaal, S. (2014). Subthreshold muscle twitches dissociate oscillatory neural signatures of conflicts from errors. NeuroImage, 86, 503-13. http://dx.doi.org/10.1016/j.neuroimage.2013.10.033
Disselhoff, V., Jakab, A., Schnider, B., Latal, B., Wehrle, F. M., & Hagmann, C. F. (2020). Inhibition is associated with whole-brain structural brain connectivity on network level in school-aged children born very preterm and at term. NeuroImage, 218, 12. http://dx.doi.org/10.1016/j.neuroimage.2020.116937
Dong, G., Wang, Z., Dong, H., Wang, M., Zheng, Y., Ye, S., Zhang, J., & Potenza, M. N. (2020). More stringent criteria are needed for diagnosing internet gaming disorder: Evidence from regional brain features and whole-brain functional connectivity multivariate pattern analyses. Journal of Behavioral Addictions, 9(3), 642-653. http://dx.doi.org/10.1556/2006.2020.00065
Gründemann, J. (2021). Distributed coding in auditory thalamus and basolateral amygdala upon associative fear learning. Current Opinion in Neurobiology, 67, 183-189. http://dx.doi.org/10.1016/j.conb.2020.11.014
Gunnell, K. E., Poitras, V. J., LeBlanc, A., Schibli, K., Barbeau, K., Hedayati, N., Ponitfex, M. B., Goldfield, G. S., Dunlap, C., Lehan, E., & Tremblay, M. S. (2019). Physical activity and brain structure, brain function, and cognition in children and youth: A systematic review of randomized controlled trials. Mental Health and Physical Activity, 16, 105-127. http://dx.doi.org/10.1016/j.mhpa.2018.11.002
Jiang, Y., Li, Z., Zhao, Y., Xiao, X., Zhang, W., Sun, P., Yang, Y., & Zhu, C. (2020). Targeting brain functions from the scalp: Transcranial brain atlas based on large-scale fMRI data synthesis. NeuroImage, 210, 14. http://dx.doi.org/10.1016/j.neuroimage.2020.116550
Kesler, S. R., Kent, J. S., & O’Hara, R. (2011). Prefrontal cortex and executive function impairments in primary breast cancer. Archives of Neurology, 68(11), 1447-1453. http://dx.doi.org/10.1001/archneurol.2011.245
Li, W., Wang, Z., Syed, S. et al. Chronic social isolation signals starvation and reduces sleep in Drosophila. Nature 597, 239–244 (2021). https://doi.org/10.1038/s41586-021-03837-0
Panikratova, Y. R., Vlasova, R. M., Akhutina, T. V., Korneev, A. A., Sinitsyn, V. E., & Pechenkova, E. V. (2020). Functional connectivity of the dorsolateral prefrontal cortex contributes to different components of executive functions. International Journal of Psychophysiology, 151, 70-79. http://dx.doi.org/10.1016/j.ijpsycho.2020.02.013
Parker, M. N., LeMay‐Russell, S., Schvey, N. A., Crosby, R. D., Ramirez, E., Kelly, N. R., Shank, L. M., Byrne, M. E., Engel, S. G., Swanson, T. N., Djan, K. G., Kwarteng, E. A., Faulkner, L. M., Zenno, A., Brady, S. M., Yanovski, S. Z., Tanofsky‐Kraff, M., & Yanovski, J. A. (2022). Associations of sleep with food cravings and loss‐of‐control eating in youth: An ecological momentary assessment study. Pediatric Obesity, 17(2), 10. http://dx.doi.org/10.1111/ijpo.12851
Romanski, L. M., & LeDoux, J. E. (1992). Equipotentiality of thalamo-amygdala and thalamo-cortico-amygdala circuits in auditory fear conditioning. The Journal of Neuroscience, 12(11), 4501-4509. https://www.proquest.com/scholarly-journals/equipotentiality-thalamo-amygdala-cortico/docview/1814153014/se-2?accountid=48841
Schienle, A., Gremsl, A., & Zorjan, S. (2022). Social reward from giving food to others affects food craving and brain potentials: An imagery-based event-related potential study. Appetite, 168, 7. http://dx.doi.org/10.1016/j.appet.2021.105722
Schmid, G., Schreier, A., Meyer, R., & Wolke, D. (2010). A prospective study on the persistence of infant crying, sleeping and feeding problems, and preschool behavior. Acta Paediatrica, 99(2), 286-290. https://www.proquest.com/scholarly-journals/prospective-study-on-persistence-infant-crying/docview/622103148/se-2?accountid=48841
Sheth, C., Furlong, T. M., Keefe, K. A., & Taha, S. A. (2017). The lateral hypothalamus to lateral habenula projection, but not the ventral pallidum to lateral habenula projection, regulates voluntary ethanol consumption.Behavioural Brain Research, 328, 195-208. http://dx.doi.org/10.1016/j.bbr.2017.04.029
Shine, J. M., Hearne, L. J., Breakspear, M., Hwang, K., Müller, E.,J., Sporns, O., Poldrack, R. A., Mattingley, J. B., & Cocchi, L. (2019). The Low-Dimensional Neural Architecture of Cognitive Complexity Is Related to Activity in Medial Thalamic Nuclei. Neuron, 104(5), 849-855.e3. http://dx.doi.org/10.1016/j.neuron.2019.09.002
Simon, E. B., Vallat, R., Barnes, C. M., & Walker, M. P. (2020). Sleep loss and the socio-emotional brain. Trends in Cognitive Sciences, 24(6), 435–450. https://www.cell.com/trends/cognitivesciences/fulltext/S1364-6613(20)30055-3?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS1364661320300553%3Fshowall%3Dtrue
Sporns, O., & Betzel, R. F. (2016). Modular brain networks. Annual Review of Psychology, 67, 613-640. http://dx.doi.org/10.1146/annurev-psych-122414-033634
St-Onge, M. P., McReynolds, A., Trivedi, Z. B., Roberts, A. L., Sy, M., & Hirsch, J. (2012). Sleep restriction leads to increased activation of brain regions sensitive to food stimuli. The American journal of clinical nutrition, 95(4), 818-824.
Tanaka, K. Z. (2021). Heterogeneous representations in the hippocampus.Neuroscience Research, 165, 1-5. http://dx.doi.org/10.1016/j.neures.2020.05.002
Todd, R. M., Miskovic, V., Chikazoe, J., & Anderson, A. K. (2020). Emotional objectivity: Neural representations of emotions and their interaction with cognition. Annual Review of Psychology, 71, 25-48. http://dx.doi.org/10.1146/annurev-psych-010419-051044
Tomova, L., Wang, K.L., Thompson, T. et al. Acute social isolation evokes midbrain craving responses similar to hunger. Nat Neurosci 23, 1597–1605 (2020). https://doi.org/10.1038/s41593-020-00742-z
Willie, J. T., Chemelli, R. M., Sinton, C. M., & Yanagisawa, M. (2001). To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annual review of neuroscience, 24(1), 429-458.
Zhu, Y., Gao, H., Tong, L., Li, Z., Wang, L., Zhang, C., Yang, Q., & Yan, B. (2019). Emotion regulation of hippocampus using real-time fMRI neurofeedback in healthy human. Frontiers in Human Neuroscience, 13, 14. http://dx.doi.org/10.3389/fnhum.2019.00242
Related Posts
Touchscreen Technology: The Next Contamination Sensor?
Figure: A water treatment facility. These are commonly found in...
Read MoreAdvancements in Regenerative Medicine – Unleashing the Potential of Artificial Organs and Organs-on-a-Chip
Source: 허동은교수, Wikimedia Commons, CC BY-SA 4.0 Introduction Recent years...
Read MoreMechanisms of Metabolic Robustness Discovered in E. Coli
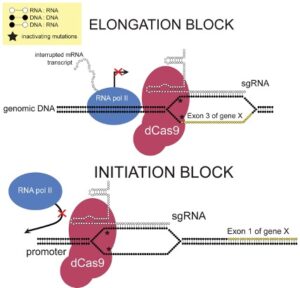
Figure 1: This diagram demonstrated how CRISPR interference (CRISPRi) works...
Read MoreThe Immunological and Physiological Effects of SARS-COV-2 on the Human Body
Figure 1: The representation of cellular activity of SARS-COV-2 as...
Read MoreProteins that Create “Speckles” in Cell Nuclei Discovered
Figure 1: A fluorescent microscopic image of the localization of...
Read More3D Bioprinting: An Innovative Approach to Promote Cartilage Regeneration
Source: Laboratoires Servier, (CC BY-SA 3.0) Many of us cannot...
Read MoreMingcong Tang