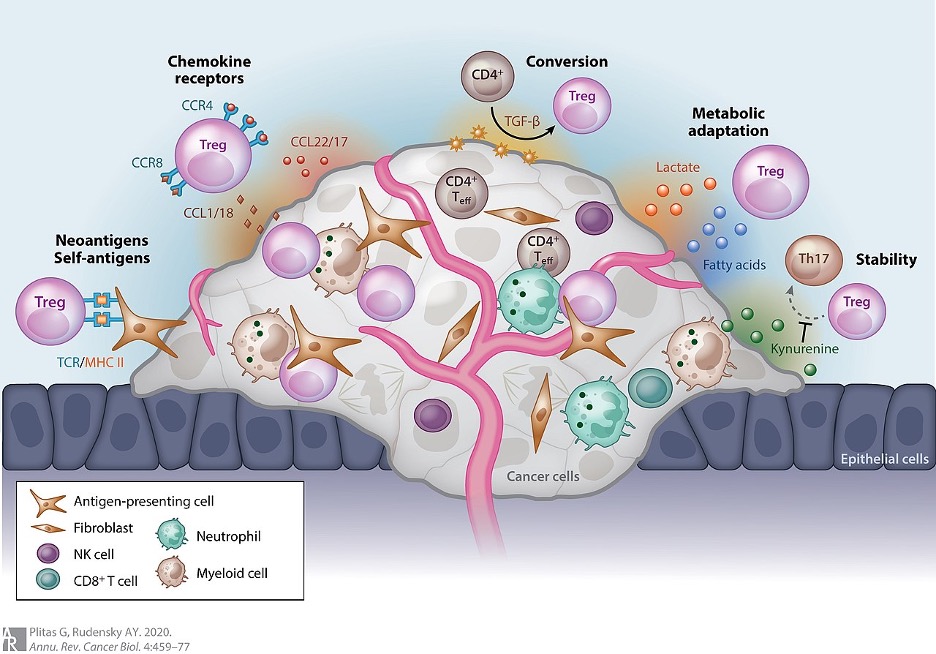
Figure 1: The different ways a tumor can provide protection for itself through Treg (seen as circular, pink cells above) recruitment
Source: Wikimedia Commons
Researchers have discovered a potential new immunotherapy for certain tumors that involves downregulating a type of immune cell called Regulatory T Cells (or Tregs) in and around the target tumor. Tregs are involved in the body’s attempt to prevent autoimmune reactions by inhibiting other immune cells that have been active for an excessively long time (prolonged immune responses often result in damage to the body due to the inflammation and large amounts cytotoxic molecules produced by immune cells) or that are attacking healthy, host cells. Tumors often recruit these regulatory cells via a variety of means (many of which can be seen in Figure 1) in order to convince the body’s immune system that is actually a non-dangerous, host tissue. This prevents immune attack and destruction of the tumor, allowing the cancer to grow. Additionally, it makes many immunotherapies directed at tumors ineffective (Sharma et al., 2017).
However, a research team at St. Jude Children’s Research Hospital has recently discovered that in many tumors, recruitment of Tregs results in differential activation of certain transcription factors in the Tregs when compared to other Tregs in the body. Inhibition of the activated transcriptional pathway resulted in fewer tumors and a stronger immune response against tumors that do appear (Lim et al., 2021).
By analyzing the contents of Tregs extracted from tumor microenvironments and those of Tregs from elsewhere in the body, Lim’s team discovered that levels of the two Sterol Regulatory Element-Binding Protein (SREBP) transcription factors and their targets were elevated in tumor-recruited cells. The SREBP proteins are responsible for upregulating the production of proteins that are involved in the synthesis of sterols. Sterols are derivatives of cholesterol used for signaling (including the hormones estrogen, testosterone, and cortisone) and are essential components of cell membranes. The fact that there was a difference in expression of these proteins in Tregs from tumors compared to Tregs elsewhere means that the proteins could serve as targets for therapies. Such a therapy could reduce the impact of tumor-shielding Tregs without allowing autoimmunity issues to arise due to the inactivation of all of the Tregs in the body (Lim et al., 2021).
To see if targeting this pathway would be an effective form of therapy, not only to attack tumors directly but also make other immunotherapies more effective, mice that possessed Tregs without SREBP-Cleavage-Activating Protein (SCAP) were produced. SCAP is a protein required for SREBP to enhance transcription of its gene targets; without SCAP, tumor-recruited Tregs would not be able to drive the expression of SREBP target genes. These Treg SCAP-deficient mice showed increased ability to fight off cancer, both alone and when given immunotherapeutic medicines. This finding was corroborated by increased amounts of Cytotoxic T Cells and Helper T Cells as well as by decreased amounts of Tregs in and around the tumors. This decrease in Tregs was likely due to decreased ability of the tumor to recruit the cells as it was detected that the loss of the SREBP pathway led to decreased ability of Tregs to survive within the tumor microenvironment (but this effect was not seen elsewhere in the body). Furthermore, the T cells were producing more of the cytotoxic molecules TNF-α and IFN-γ (Lim et al., 2021). A decrease in IFN-γ secretion is common within tumors that have recruited Tregs, a result known as “fragility” that likely contributes to the increased survivability of Treg-protected tumors (Overacre-Delgoffe et al., 2017). An increase in these molecules may have helped the modified mice to fight off cancer.
While this finding was really encouraging, the researchers had to be sure that the deletion of SCAP did not affect the Tregs of the mice in such a way so as to decrease their ability to prevent autoimmune reactions. Luckily, this was not the case. Normal levels of Tregs, Helper T Cells, and Cytotoxic T Cells throughout the body were detected and no increase in autoimmune diseases or inflammation was seen in the modified line of mice over time (Lim et al., 2021).
Additionally, it is known that the sterols produced by the SREBP pathway are required for the proliferation of Cytotoxic T Cells in response to activation by a pathogen (Kidani et al., 2013). Based on these findings, the researchers predicted that this could possibly affect the rate of proliferation of Tregs as well. Unfortunately, this side-effect of the treatment was seen and fewer Tregs were produced upon stimulation. However, this effect was easily mitigated by cholesterol (a precursor for the needed sterols) supplementation (Lim et al., 2021).
Based on these findings, developing a small molecule that can target the SREBP pathway within Tregs could provide an incredibly effective and safe way of preventing and fighting tumors as well as enhancing the effects of other immunotherapies.
References
Kidani, Y., Elsaesser, H., Hock, M. B., Vergnes, L., Williams, K. J., Argus, J. P., Marbois, B. N., Komisopoulou, E., Wilson, E. B., Osborne, T. F., Graeber, T. G., Reue, K., Brooks, D. G., & Bensinger, S. J. (2013). Sterol regulatory element-binding proteins are essential for the metabolic programming of effector T cells and adaptive immunity. Nature Immunology, 14(5), 489–499. https://doi.org/10.1038/ni.2570
Lim, S. A., Wei, J., Nguyen, T.-L. M., Shi, H., Su, W., Palacios, G., Dhungana, Y., Chapman, N. M., Long, L., Saravia, J., Vogel, P., & Chi, H. (2021). Lipid signalling enforces functional specialization of T reg cells in tumours. Nature, 1–6. https://doi.org/10.1038/s41586-021-03235-6
Overacre-Delgoffe, A. E., Chikina, M., Dadey, R. E., Yano, H., Brunazzi, E. A., Shayan, G., Horne, W., Moskovitz, J. M., Kolls, J. K., Sander, C., Shuai, Y., Normolle, D. P., Kirkwood, J. M., Ferris, R. L., Delgoffe, G. M., Bruno, T. C., Workman, C. J., & Vignali, D. A. A. (2017). Interferon-γ Drives Treg Fragility to Promote Anti-tumor Immunity. Cell, 169(6), 1130-1141.e11. https://doi.org/10.1016/j.cell.2017.05.005
Sharma, P., Hu-Lieskovan, S., Wargo, J. A., & Ribas, A. (2017). Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell, 168(4), 707–723. https://doi.org/10.1016/j.cell.2017.01.017
St. Jude Children’s Research Hospital. (2021). Discovery offers potential for stripping tumors of T cell protection. ScienceDaily. www.sciencedaily.com/releases/2021/02/210224120316.htm
Related Posts
COVID-19 and its Implications for Adolescent Mental Health
Covid 19 and Isolation (Source: created by author) Abstract The...
Read MoreCovid-19 and the Next Pandemic: Designing a Science-based Strategy to Prepare for the Next Pandemic
Taking the proper, science-backed steps could help prevent the next...
Read MoreInnovation Beyond All Barriers: The Arrival of a Life-Saving Treatment
Figure 1:Magnetic resonance image (MRI) – coronal slice of a...
Read MoreFrankie Carr