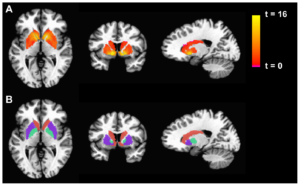
Figure 1: Neural imaging of the default mode network (pictured bottom left) with two key sub-regions identified, the mPFC and PCC
Source: Wikimedia Commons
Post-Traumatic Stress Disorder (PTSD) can be defined as a mental health condition which has been triggered by experiencing or witnessing traumatic events. Trauma can have lasting effects on one’s sense of self (Schore, 2003) – on both cognitive (related to thinking) and somatic (related to voluntary movement) brain function (Lanius, Frewen, Tursich, Jetly, & McKinnon, 2015; for an extensive review article, see Frewen et al., 2008, 2020).
During PTSD, individuals are distressed by negative beliefs about themselves, which can include thoughts like: ‘I will never be able to feel normal emotions again,’ ‘I feel like an object, not like a person,’ ‘I do not know myself anymore,’ or, ‘I have permanently changed for the worse’ (Cox, Resnick, & Kilpatrick, 2014; Foa, Tolin, Ehlers, Clark, & Orsillo, 1999).These thoughts are often accompanied by physical issues such as lower back pain, general muscle aches and pains, or excessive flatulence/burping. These somatic disturbances can all significantly perturb one’s self-image (Graham, Searle, Van Hooff, Lawrence-Wood, & McFarlane, 2019).
The Default Mode Network (DMN) is a system of connected brain areas that show increased activity when a person is not focused on the outside world. In other words, it is a neurological structure which is activated when the brain is at ‘rest’. During rest, the DMN is active and the brain uses less energy. The DMN is composed primarily of the cortical regions located across the brain’s mid-line, including the posterior cingulate cortex, the precuneus, and the medial prefrontal cortex (Spreng, Mar, & Kim, 2009; Greicius et al., 2003; Buckner, Andrews-Hanna, & Schacter, 2008; for a review, see Qin & Northoff, 2011).
The Default Mode Network (DMN) is known to be atypically active during PTSD when an individual engages in introspective activities such as daydreaming, contemplating the past or the future, or thinking about the perspective of someone else. Research shows that childhood maltreatment may affect development of the DMN, which begins maturation during childhood and does not end until early adulthood (Sherman et al., 2014; for a review, see Fair et al., 2008). If the trauma(s) is repeated, the DMN will be biased towards the trauma and can re-experience or relive it as long as the trauma remains unprocessed.
The DMN can be divided into two major subsystems – the Medial Prefrontal Cortex (mPFC) and the Medial-Temporal Lobe (MTL). These subsystems are recruited during autobiographical memory processing – or processing memories of one’s own personal experience (Buckner et al., 2008; Kim, 2012; Philippi, Tranel, Duff, & Rudrauf, 2013). However, during PTSD there is an absence of specific detail in autobiographical memory. Individuals with PTSD have difficulty recalling unique memories and tend to produce memories along the lines of “it was raining that day,” instead of, “when I was driving back home from work on Wednesday, it started to rain suddenly.” This phenomenon is referred to as Overgeneral Autobiographical Memory (OGAM).
The Medial Prefrontal Cortex (mPFC) of the DMN is an area at the medial wall of the anterior frontal lobes. Within this area are dense white matter connections between the ventral region of the mPFC (vmPFC) and amygdala, facilitating bidirectional communication between them (Aggleton et al., 1980; Stefanacci and Amaral 2002). Researchers have shown that the volitional suppression of negative emotion in healthy adults is associated with an inverse relationship between vmPFC and amygdala activity (Delgado et al., 2008; Johnstone et al., 2007; Urry et al., 2006). Specifically, there is an increase in vmPFC activity associated with a decrease in amygdala activity as well as a decrease in the experience of negative affect (i.e. negative emotion). This vmPFC-amygdala coupling during emotion regulation is disrupted in patients with major depressive disorder (Johnstone et al., 2007), which is characterized by pathologically high levels of negative affect.
Studies show that vmPFC lesion patients display diminished self-insight (reduced capability to understand oneself) and self-monitoring (the ability to evaluate personal behavior) (Barrash et al., 2000; Beer et al., 2006). Moreover, vmPFC patients exhibit the self-critical emotions of shame, guilt, embarrassment, and regret at high frequency (Beer et al., 2006; Camille et al., 2004; Koenigs et al., 2007). Furthermore, data from another recent study indicates that vmPFC lesion patients also experience “somatic” symptoms of depression including fatigue and changes in appetite (Koenigs et al., 2008).
The Medial Temporal Lobe (MTL) subsystem of the DMN is particularly engaged when participants generate memory episodes specific in time and place and is sensitive to scene construction (i.e. the need to embed an event within a spatial context) (Andrews-Hanna et al., 2010; Addis et al., 2011; Madore et al., 2016; Tamir et al., 2016; Palombo et al. 2018). The MTL subsystem is comprised of the hippocampus, para hippocampus, retro splenial cortex, posterior inferior parietal lobule and, to some extent, the ventral medial prefrontal cortex (vMPFC).
Researchers have found that PTSD was associated with alterations in connectivity within the MTL subsystem. These alterations in the MTL subsystem may contribute to impairments in contextual fear extinction processes in PTSD, as the hippocampus is involved in contextual fear extinction recall and is less active during fear extinction recall in PTSD.
Researchers have also noticed that the PCC and hippocampus are functionally connected, and that this connection is associated with avoidance/numbing symptoms which includes avoidance of thoughts and feelings associated with the trauma, avoidance of reminders of the trauma, or inability to recall an important aspect of the trauma.
In sum, these studies document the importance of the MTL subsystem in avoidance symptoms during PTSD. It has been observed that the alterations in connectivity between the PCC and the MTL subsystem of the DMN also disrupt autobiographical memory processing and thereby contribute to the symptom of PTSD. Given all of the above research findings, the Default Mode Network (DMN) and its subsystems clearly play an integral role in producing and modulating PTSD symptoms. The medial Prefrontal Cortex (mPFC) evokes guilt, shame and regret, whereas the Medial Temporal Lobe (MTL) is responsible for avoidance and numbing. Researchers have recommended that different types of psychotherapy such as Cognitive Processing Therapy, Prolonged Exposure Therapy, Stress Inculcation Training and meditation may lead to recovery of individuals with PTSD.
References
Lanius, Ruth A., Braeden A. Terpou & Margaret C. McKinnon. (2020). The sense of self in the aftermath of trauma: lessons from the default mode network in post-traumatic stress disorder, European Journal of Psychotraumatology 11(1). 1807703. DOI: 1080/2000819888.2020.1807703.
Schore, Allan N. (2003). After Dysregulation & Disorders of the self. W.W. Norton &Company.
Lanius, R. A., Frewen, P. A., Tursich, M., Jetly, R., & McKinnon, M. C. (2015). Restoring large-scale brain networks in PTSD and related disorders: A proposal for neuroscientifically-informed treatment interventions. European Journal of Psychotraumatology, 6(1), 1–12.
Frewen, P. A., Schroeter, M. L., Riva, G., Cipresso, P., Fairfield, B., Padulo, C., & Northoff, G. (2020). Neuroimaging the consciousness of self: Review, and conceptual-methodological framework. Neuroscience and Biobehavioral Reviews, 112, 164–212.
Foa, E. B., Tolin, D. F., Ehlers, A., Clark, D. M., & Orsillo, S. M. (1999). The posttraumatic cognitions inventory (PTCI): Development and validation. Psychological Assessment, 11(3), 303–314.
Foa, E. B., Tolin, D. F., Ehlers, A., Clark, D. M., & Orsillo, S. M. (1999). The posttraumatic cognitions inventory (PTCI): Development and validation. Psychological Assessment, 11(3), 303–314.
Spreng, R. N., Mar, R. A., & Kim, A. S. N. (2009). The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta-analysis. Journal of Cognitive Neuroscience, 21(3), 489–510.
Greicius, M. D., Krasnow, B., Reiss, A. L., & Menon, V. (2003). Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the USA, 100(1), 253–258.
Buckner, R. L., Andrews-Hanna, J. R., & Schacter, D. L. (2008). The brain’s default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences, 1124(1), 1–38.
Qin, P., & Northoff, G. (2011). How is our self-related to midline regions and the default-mode network? Neuroimage, 57(3), 1221–1233.
Sherman, L. E., Rudie, J. D., Pfeifer, J. H., Masten, C. L., McNealy, K., & Dapretto, M. (2014). Development of the default mode and central executive networks across early adolescence: A longitudinal study. Developmental Cognitive Neuroscience, 10, 148–159.
Fair, D. A., Cohen, A. L., Dosenbach, N. U. F., Church, J. A., Miezin, F. M., Barch, D. M., & Schlaggar, B. L. (2008). The maturing architecture of the brain’s default network. Proceedings of the National Academy of Sciences of the USA, 105(10), 4028–4032.
Buckner, R. L., Andrews-Hanna, J. R., & Schacter, D. L. (2008). The brain’s default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences, 1124(1), 1–38.
Kim, H. (2012). A dual-subsystem model of the brain’s default network: Self-referential processing, memory retrieval processes, and autobiographical memory retrieval. NeuroImage, 61(4), 966–977.
Philippi, C. L., Tranel, D., Duff, M., & Rudrauf, D. (2013). Damage to the default mode network disrupts autobiographical memory retrieval. Social Cognitive and Affective Neuroscience, 10(3), 318–326.
Mayo Clinic. (6 July 2018). Post-Traumatic Stress Disorder, Symptoms and Causes.
Danielle R. Miller, Scott M. Hayes, Jasmeet P. Hayes, Jeffrey M. Spielberg, Ginette Lafleche, and Mieke Verfaellie. (2017). Default Mode Network Subsystems are Differentially Disrupted in Posttraumatic Stress Disorder. Biological psychiatry. Cognitive Neuroscience and Neuroimaging. 2(4):363-371. DOI: 10.1016/j.bpsc.2016.12.006.
U.S. Department of Veterans Affairs.Biol Psychiatry Cogn Neurosci Neuroimaging. 2017 May; 2(4): 363–371. doi: 10.1016/j.bpsc.2016.12.006
Michael Koenigs and Jordan Grafman. Post-traumatic stress disorder: The role of medial prefrontal cortex and amygdala. Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta).
Aggleton JP, Burton MJ, Passingham RE. Brain Res. 1980 May 26; 190(2):347- 68.
Beer JS, John OP, Scabini D, Knight RT. Orbitofrontal cortex and social behavior: integrating self-monitoring and emotion-cognition interactions. J Cogn Neurosci. 2006 Jun; 18(6):871-9.
Koenigs M, Huey ED, Calamia M, Raymont V, Tranel D, Grafman J (2008). J Neurosci. Distinct regions of prefrontal cortex mediate resistance and vulnerability to depression. 28(47):12341-8.
Delgado, MR, Nearing, KI, LeDoux, JE & Phelps, EA. (2008) Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron 59(5): 829-838
Delgado MR, Nearing KI, Ledoux JE, Phelps EA.Neuron. 2008 Sep 11; 59(5):829-38. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression.
Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ
J Neurosci. 2007 Aug 15; 27(33):8877-84.
Stefanacci L, Amaral DG, J Comp Neurol. 2002 Sep 30; 451(4):301-23.
Some observations on cortical inputs to the macaque monkey amygdala: an anterograde tracing study.
Psychology Today. (16 January 2020). What is Default Mode Network?
https://www.psychologytoday.com/us/basics/default-mode-network
Related Posts
Emotion-Related Impulsivity and Disordered Eating Behaviors: A Literature Review
Figure: An artistic depiction of disordered eating behavior under the...
Read MoreWhy Future A.I Robots Should Be Created With Immutable Code Limiting Singularity
Figure: Sophia, the first robot citizen in the Global A.I...
Read MoreRecent Study Conducted by Duke University Researchers Reveals Critical Limitation of fMRI Measurements
Figure 1: fMRI is used to display differences in basal...
Read MoreDrishti Hajong