Figure 1: The microbiota-gut-brain axis is a bidirectional pathway of communication between the enteric and central nervous systems. Shared abnormalities in MGB axis signaling may explain the comorbid overlaps between ASD and AN
Source: created by the author via BioRender
Introduction
The etiological paradigm for neurological disorders is currently shifting to one centered less on the brain and more on the gut. High frequencies of gastrointestinal and immune comorbidities in patients with conditions such as depression, autism, and schizophrenia imply a role for the microbiota-gut-brain (MGB) axis (a nervous, immune, and endocrine communication system) in the pathogenesis of various mental disorders. Frequent overlaps amongst neurodevelopmental (autism), mood (anxiety and depression), feeding (anorexia nervosa), autoimmune, social impairment, and sensory processing disorders might be explained by a shared pathogenesis related to MGB axis dysfunction. While the exact role of the MGB axis in such disorders is uncertain, multiple hypotheses have been proposed.
Identifying commonalities between MGB axis hypotheses about various neurological, immune, and psychiatric conditions may lead to the best pathophysiological explanations for these disorders. For example, autism spectrum disorder (ASD) and anorexia nervosa (AN) often co-occur and share multiple comorbid symptoms. This article proposes that this overlap might be explained by shared MGB axis-related etiologies. Furthermore, these possible shared mechanisms for ASD and AN may inform next steps for research efforts pertaining to these disorders.
MGB axis dysfunction as a pathophysiological mechanism of neurological disorder etiology
The MGB axis is a bidirectional communication system of nervous, immune, and endocrine pathways by which the enteric nervous system (ENS) of the gastrointestinal (GI) tract and the central nervous system (CNS) regulate the development and function of one another (Liang et al., 2018). Previously known as the gut-brain axis, this network has been recently renamed to reflect the role of the intestinal microbiome, which comprises 90-95% of cells in the gut, as a key mediator of gut-brain communication (Liang et al., 2018). Studies on animals with altered or eliminated gut microbiomes have demonstrated the importance of this microbiome to the functioning of a breadth of physiological systems, including pain perception and response, memory, mood (anxiety and depression), temperament, stress response, appetite, immune development and regulation, metabolism, sensory-motor processing, and social interaction (Liang et al., 2018; Arresti Sanz and El Aidy, 2019; van de Wouw et al., 2017; Oleskin et al., 2016; Fields et al., 2018; Johnson, 2020). Given the overwhelming evidence for the involvement of the gut microbiome in various processes moderated by the MGB axis, it is unsurprising that an altered gut microbiota would lead to various systemic dysfunctions, including those of the brain (Liang et al., 2018). Indeed, several sources confirm the role of the microbiome in the development of various neurological disorders, including depression, autism, and schizophrenia (Liang et al., 2018; Obrenovich, 2018; Rudzki and Szulc, 2018; Mangiola et al., 2016). While the association between an altered gut microbiota (dysbiosis) and neurological and mood disorders is broadly accepted, the precise mechanisms producing this relationship are unclear, especially given the bidirectional nature of the MGB axis.

Figure 2: The branches of the nervous system. The MGB axis coordinates the activities of the CNS and ENS
Source: Wikimedia Commons
The three predominant theories of dysbiosis pathogenicity are the gut-microbiota hypothesis, the “old friends”/early immune challenge hypothesis, and the leaky gut theory; each centers on the role of the gut microbiota in neurological, immune, and/or endocrine dysfunction (Liang et al., 2018).
The gut-microbiota hypothesis
Proposed by Liang and colleagues, the gut-microbiota hypothesis states that modern lifestyle factors (such as a more processed diet, reduced exercise, increased hygienic practices) alter the gut microbiota to yield one much different from that which was so carefully tailored through evolution (2018). Because the microbiota is so intricately linked to the development and function of the CNS, dysbiosis leads to neurological dysfunction in the form of mental illness, which has also risen over time in parallel with the aforementioned lifestyle changes. According to this hypothesis, the underlying cause of many mental illnesses is lifestyle-driven dysbiosis, which can be remedied with dietary or supplementary pre- and probiotics (Liang et al., 2018).
The “old friends” hypothesis
Rook and Lowry’s “old friends” hypothesis (2008) is similarly hinged on the concept that certain gut microbes, referred to as “old friends,” have evolved symbiotically with the human race; modern departures from previous lifestyles harm the “friends,” whose presence is essential for proper immune system development, thereby leading to immune dysfunction (Liang et al., 2018). For example, during development, the exposure of dendritic cells to certain species of the gut microbiota is essential for the maturation of T-lymphocytes; without proper microbiota composition, T-lymphocytes differentiate into effective rather than regulatory T-lymphocytes which prevents proper regulation of the immune response (Liang et al., 2018). The microbiota is also known to regulate the development of the gut-associated lymphoid tissue (GALT), as well as the complement system, a proteinaceous immune complex involved in various neurodevelopmental processes as well as antigen destruction (Mangiola et al., 2016; Rudzki and Szulc, 2018). The “old friends” hypothesis states that dysbiosis-related dysregulation of the immune system explains the rise in autoimmune disorders, allergies, chronic inflammation, and mental illnesses alongside modernization (Liang et al., 2018). Notably, high levels of immunoglobulins against dietary peptides and/or bacterial antigens have been repeatedly reported in the serum of patients with neurological disorders, including ASD, schizophrenia, and depression, implying that an inappropriate immune response is common to such conditions; this further supports the “old friends” hypothesis (Rudzki and Szulc, 2018).
The leaky gut theory
The leaky gut theory departs from the previous two hypotheses by instead focusing on the role of the MGB axis in the development and maintenance of the gastrointestinal-blood barrier (GBB) and blood-brain barrier (BBB) (Liang et al., 2018). Microbes in the gut produce short-chain fatty acids (SCFAs) which stimulate intestinal epithelial regeneration and mucous production to strengthen the GBB; without proper microbiome composition, SCFA production is reduced, and the integrity of the intestinal barrier is compromised (Mangiola et al., 2016). Moreover, altered SCFA levels are associated with an inflammatory immune response: it has been experimentally demonstrated that excess circulating pro-inflammatory cytokines (which can be triggered by dysbiosis directly or by SCFA alterations) can further contribute to intestinal and BBB permeability (Rudzki and Szulc, 2018; Garcia-Gutierrez et al., 2020). Dysbiosis can also increase circulation of pro-inflammatory bacterially-derived lipopolysaccharides (LPS), which enter the bloodstream due to leaky gut and cause further systemic immune response including neuroinflammation and cytokine secretion (leading to even more GBB and BBB permeability) (Liang et al., 2018; Mangiola et al., 2016; Rudzki and Szulc, 2018; Garcia-Gutierrez et al., 2020; van de Wouw et al., 2017).
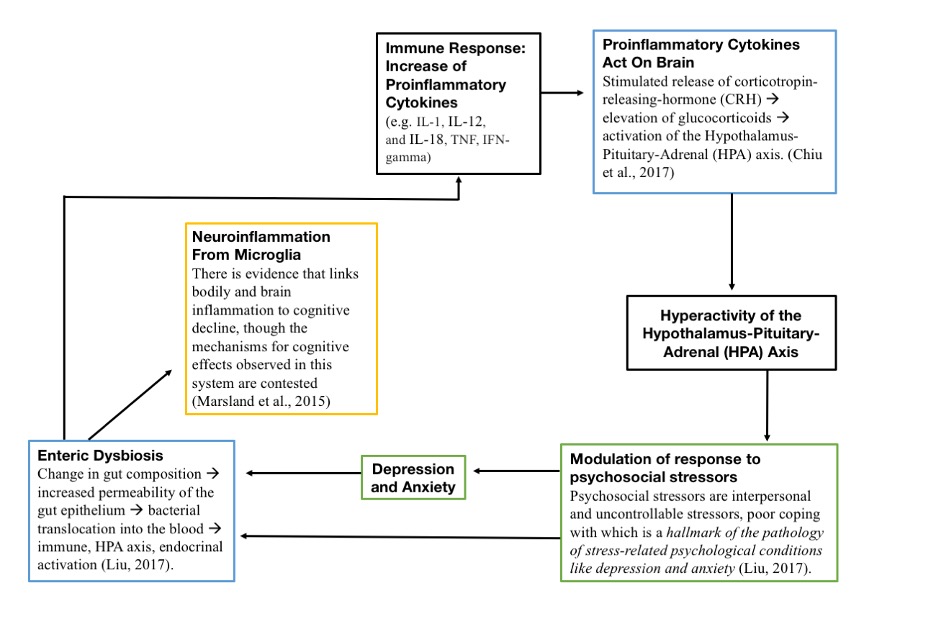
Figure 3: A schematic of dysbiosis-induced neuroinflammation and abnormal stress response. In a cycle where the initial cause of dysfunction is still unclear, it is thought that gut dysbiosis leads to intestinal permeability, allowing bacterial metabolites to circulate in the bloodstream. This then induces neuroinflammation, systemic immune response, and altered HPA axis stress response, which perpetuates dysbiosis
Source: Wikimedia Commons
Loss of GBB and BBB integrity and neuroinflammation are both associated with various neurological disorders, including depression, schizophrenia, ASD, and AN (Garcia-Gutierrez et al., 2020; Liang et al., 2018; Obrenovich, 2018; Rudzki and Szulc, 2017; Gibson and Mehler, 2018; Lobzhanidze et al., 2019; Seitz et al., 2019; Fields et al., 2018; van de Wouw et al., 2017; Karakula-Juchnowicz et al., 2017). In addition to causing BBB permeability and neuroinflammation, elevated circulating cytokines and bacterial metabolites have been shown to directly alter brain activity, further supporting the hypothesis that dysbiosis-induced leaky gut can cause brain dysfunction (Mangiola et al., 2016; Obrenovich, 2018; Liang et al., 2018; Liu and Zhu, 2018; Aresti Sanz and El Eidy, 2019).
MGB axis dysfunction as a mechanism of ASD pathogenesis
One of the fastest growing areas of MGB axis research is focused on its implications for the etiology of autism spectrum disorder (ASD). ASD is a neurodevelopmental disorder marked by stereotyped and repetitive behaviors, impaired social communication, and sensory processing disorders (Israelyan and Margolis, 2019; Galiana-Simal et al., 2017). While the prevalence of ASD in the United States is as high as 1 in 54, the etiology of the disorder is still unclear, in part due to its heterogeneity of presentation (CDC, 2020; Israelyan and Margolis, 2019; Vissoker et al, 2015; Sanctuary et al, 2018; Xu et al, 2019; Azhari et al, 2018).
A growing body of neurological, immunological, and gastrointestinal-based theories points to the potential role of the MGB axis and gut dysbiosis in ASD pathogenesis (Azhari et al., 2018; Srikantha and Mohajeri, 2019; Fattorusso et al., 2019; Tye et al., 2018). This is supported by the high frequency of gastrointestinal and immunological comorbidities in children with ASD. The literature reports a four times greater prevalence of GI comorbidities in children with ASD than without, and the common comorbidity of constipation has been found to correlate with symptoms like social impairment, aggression, and compulsivity (Sanctuary et al., 2018; Israelyan and Margolis, 2019; Fattorusso et al., 2019). Additionally, the correlation between ASD severity and immune dysregulation (food allergies, asthma, and systemic inflammation evidenced by upregulated proinflammatory cytokines, natural killer cells, interferons, and autoantibodies) further points to the potential role of MGB axis dysfunction (Tye et al., 2018; Vissoker et al., 2015; Azhari et al., 2018; Fattorusso et al., 2019). Thus, a complex phenotypic pattern of gastrointestinal, immunological, and neurological dysfunction has been identified, suggesting a connection between ASD and MGB axis dysfunction (although a causal relationship is yet to be established by human studies) (Azhari et al., 2018; Srikantha and Mohajeri, 2019; Fattorusso et al., 2019; Xu et al., 2019). The induction of ASD-associated traits (such as repetitive behaviors and related social and cognitive dysfunctions) in mice through microbiome manipulation demonstrates that gut dysbiosis indeed plays a role in ASD, and trials of Microbiota Transfer Therapy show promise for improving ASD symptoms and GI comorbidities; thus, it is worthwhile to continue to investigate the patterns, mechanisms, and causal relationships underlying this connection, especially within the context of human ASD patients (Kang et al., 2017; Wang et al., 2019; Liang et al., 2018; Srikantha and Mohajeri, 2019; Rudzki and Szulc, 2018; Mangiola et al., 2016; Sanctuary et al., 2018; Fattorusso et al., 2019).
While GI disorders do not present in all cases of ASD, and neither do autoimmune and anxiety disorders, the correlation between these three comorbidities in a subset of cases leads some to believe that there is a particular immune-mediated subtype of ASD distinguished by degree of systemic inflammation (Tye et al., 2018; Fattorusso et al., 2019; Azhari et al., 2018). Given prior research, it seems likely that this subtype might be found in patients with co-occurring ASD, GI disorders, and immunological disorders; this is explained by Azhari and colleagues’ gut-immune-brain paradigm (2018).
The “gut-immune-brain” paradigm of ASD
One hypothesis grounded in MGB axis dysfunction is the “gut-immune-brain” paradigm, proposed by Azhari and colleagues (2018). Featuring parts of the gut-microbiota, old friends, and leaky gut theories, the paradigm states that gut dysbiosis leads to the autistic phenotype and its comorbidities through four interacting mechanisms: an increase in intestinal permeability (“leaky gut”), toxin production and exposure, aberrant immune response, and metabolic abnormalities (Azhari et al., 2018). This theory is supported by repeated findings of dysbiosis, abnormal levels of circulating bacterial metabolites, immune dysregulation, and impaired metabolic processes in association with ASD (Fattorusso et al., 2019; Israelyan and Margolis, 2019; Sanctuary et al., 2018; Srikantha and Mohajeri, 2019; Obrenovich, 2018; Rudzki and Szulc, 2018; Mangiola et al., 2016; Dempsey et al., 2019; Shultz et al., 2014; Garcia-Gutierrez et al., 2020; Nankova et al., 2014; MacFabe, 2012; Xu et al., 2019; Fields et al., 2018; Lobzhanidze et al., 2019). Given differential immune responses between males and females (for example, males exhibiting a more pro-allergic response), an immune-driven paradigm of ASD could also explain the overrepresentation of males compared to females in the autistic population (Fields et al., 2018; Werling and Geschwind, 2014). Furthermore, the explanatory power of the paradigm lies in its application to many of the leading proposed mechanisms of ASD and its ability to account for their interactions such as through positive feedback loops.
It is important to note that stress alone has been demonstrated to alter the gut microbiome as well as gut permeability, and so one might argue that a genetic and/or environmental predisposition to barrier permeability and/or immuno-inflammatory responses could cause enough physiological stress to induce dysbiosis (Liu and Zhu, 2018; Rudzki and Szulc, 2018; Liang et al., 2018). In this case, it is possible that dysbiosis is merely correlated with the immune response but does not cause it in the first place. However, dietary intervention, probiotic, and fecal transplantation trials demonstrating amelioration of stereotyped behaviors and comorbidities in ASD suggest that a causal relationship might indeed exist between dysbiosis and ASD pathogenesis (Rudzki and Szulc, 2018; Mangiola et al., 2016; Sanctuary et al., 2018; Srikantha and Mohajeri, 2019; Fattorusso et al., 2019).
Patterns of dysbiosis in ASD
While studies on ASD patient microbiomes have yielded heterogenous and at times conflicting results, a number of significant patterns have emerged. Analyses of the fecal microbiota of children with ASD compared with typical development peers and/or siblings reveals that Bacteroidetes, Clostridium, Desulfovibrio, Faecalibacterium, Proteobacteria (including Sutterella), Lactobacillus, and Actinobacteria are overrepresented in ASD; Bifidobacterium, Prevotella, Coprococcus, and Veillonellaceae are lacking, and overall species diversity is diminished as well (Fattorusso et al., 2019; Tye et al., 2018; Nankova et al., 2014, Azhari et al., 2018; Garcia-Gutierrez et al., 2020; Sanctuary et al., 2018; Fields et al., 2018; Oleskin et al., 2016; Xu et al., 2019; Wang et al., 2019; Dempsey et al., 2019). Additionally, higher Firmicutes: Bacteroidetes ratios have been noted in ASD (Srikantha and Mohajeri, 2019; Sanctuary et al., 2018). While no definite ASD microbiome profile has emerged, the Human Microbiome Project has demonstrated that the microbiomes of two healthy humans can vary greatly in comparison to one another, highlighting that perhaps it is not the presence of an ideal profile, but the balance between competing and symbiotic species that demarcates a healthy gut from dysbiosis (Fattorusso et al., 2019; Israelyan and Margolis, 2019; Fields et al., 2018). As van de Wouw and colleagues note, a less diverse gut microbiome might allow one species to dominate, influencing the host in ways otherwise impossible; additionally, a variety of dysbiotic profiles could disrupt the microbial ecosystem by hampering cross-feeding (the supplying of nutrients for one species by the metabolites of another). This is particularly relevant to SCFA production, a mechanism of interest for gut-related neurological disorder pathogenesis (2017).
Bacteroidetes, Clostridium, and Desulfovibrio-produced propionate as a mechanism of ASD
There are several hypotheses pertaining to certain species that are found in elevated or diminished abundance which could explain the correlation between dysbiotic patterns in ASD and the etiology of the disorder. For example, the lack of Bacteroidetes, Clostridium, and Desulfovibrio in ASD patients lends support to a propionate-related theory of ASD pathogenesis (Garcia-Gutierrez et al., 2020; Nankova et al., 2014; MacFace, 2012). It is known that the aforementioned bacteria produce propionate (PPA), one of the three primary SCFAs, and the overgrowth of these species in ASD correlates with elevated levels of PPA in stool (Dempsey et al., 2019; Shultz et al., 2014; Lobzhanidze et al., 2019; Nankova et al., 2014; Srikantha and Mohajeri, 2019; Garcia-Gutierrez et al., 2020; Fields et al., 2018). It is known that PPA can cross the BBB, triggering an immune response; chronic PPA administration in mice is correlated with neuroinflammatory biomarkers (Garcia-Gutierrez et al., 2020; Lobzhanidze et al., 2019). There is also evidence that excess PPA exposure impacts amygdala development and function; this is pertinent to ASD as social communication and emotional processing is often impaired in the disorder (particularly gaze, attachment behavior, and social behavior) (Lobzhanidze et al., 2019). PPA exposure in mice decreases social motivation, reduces neuron number in the amygdala, and activates glial cells, causing further inflammation (Lobzhanidze et al., 2019). Although the data is currently inconclusive, there is evidence that structural changes of the amygdala correlate with degree of social impairment in ASD; the impacts of PPA on the amygdala could explain this association (Lobzhanidze et al., 2019). Furthermore, PPA is a rather convincing mechanism of ASD as murine modeling has shown an association between PPA and an array of ASD-related traits and comorbidities such as reduced food intake, hyperactivity, repetitive behaviors, obsessive/compulsive behaviors, mood disorders, impaired social behavior, altered fatty acid metabolism, altered BBB integrity, reduced gastric motility, developmental delay, GI dysfunction, immune dysfunction, and abnormal gastrointestinal serotonin secretion (Dempsey et al., 2019; Garcia-Gutierrez et al., 2020; Nankova et al., 2014; MacFabe, 2012; Lobzhanidze et al., 2019).
Faecalibacterium overgrowth as a cause of ASD-related immune dysfunction
Faecalibacterium is often overrepresented in the gut microbiomes of ASD patients (Dempsey et al., 2019; Garcia-Gutierrez et al., 2020; Xu et al., 2019; Azhari et al., 2018). This trait has been associated with systemic immune dysfunction (a common comorbidity of ASD), which is the result of altered butyrate (BA) production, where BA is a known metabolite of Faecalibacterium (Xu et al., 2019; Valles-Colomer et al., 2019). Some studies have measured high BA as correlated with high Faecalibacterium in ASD (Dempsey et al., 2019; Garcia-Gutierrez et al., 2020). This could also explain other comorbidities of ASD associated with high BA such as satiety/reduced intake, indigestion, constipation, altered social behavior, and immune dysfunction (Garcia-Gutierrez et al., 2020; Lin et al., 2012). However, the data and ensuing conclusions on the role of BA in ASD are far from conclusive: other studies found reduced BA in ASD and explained impaired GBB integrity as the intestinal mucosa is constructed from SCFAs including BA (Lobzhanidze et al., 2019; Srikantha and Mohajeri, 2019; Valles-Colomer et al., 2019). While different studies have found either increased or decreased levels of BA in ASD patients, it seems likely that either way, a BA imbalance contributes to ASD etiology. As a key SCFA, BA is implicated in other SCFA-related processes relevant to ASD and its comorbidities, such as serotonin production and secretion, inflammatory immune response, intestinal mucous production, vagal signaling, neurodevelopment, appetite regulation (via secretion of anorexigenic hormones GLP-1 and PYY), metabolism, gene expression (via histone acetylation and methylation), BBB maintenance, and lipid metabolism (Mangiola et al., 2016; Dempsey et al., 2019; Garcia-Gutierrez et al., 2020; Yang et al., 2019; Nankova et al., 2014, MacFabe, 2012; Oleskin et al., 2016; van de Wouw et al., 2017; Fields et al., 2018; Nankova et al., 2014).
Proteobacteria overgrowth as a cause of ASD-related immune dysfunction through LPS and TNF-ɑ
Proteobacteria, specifically Sutterella, have been found at elevated numbers in ASD patients and might contribute to ASD pathogenesis in line with the gut-immune-brain paradigm (Azhari et al., 2018; Fattorusso et al., 2019; Tye et al., 2018; Fields et al., 2018). An increase in Proteobacteria is associated with metabolic endotoxemia (low-grade systemic inflammation) through the overproduction and circulation of lipopolysaccharides (LPS) (van de Wouw et al., 2017; Fields et al., 2018). LPS interacts with toll-like receptor 4 (TLR4) to increase gut permeability, leading to a cascade of effects on CNS function, including altered SCFA circulation, vagal signaling, stress response and inflammatory immune response (Fields et al., 2018; Garcia-Gutierrez et al., 2020; Xu et al., 2019; Karakula-Juchnowicz et al., 2017). In mice, increased levels of circulating LPS are associated with repetitive behaviors, underscoring the implications of LPS for not only ASD comorbidities but also for hallmark traits (Fields et al., 2018).
Furthermore, LPS stimulates the secretion of TNF-ɑ from liver cells, leading to peripheral inflammation, inhibition of INF-β (which is essential for the intestinal mucosa lining), and impairment of tight junction formation in the GBB and BBB, thereby further perpetuating intestinal and brain permeability and systemic inflammation (Azhari et al., 2018; Karakula-Juchnowicz et al., 2017). Notably, TNF-ɑ correlates with the severity of ASD symptoms in general and GI dysfunction specifically (Garcia-Gutierrez et al., 2020; Srikantha and Mohajeri, 2019). The molecule is thought to influence CNS function by crossing the BBB, inducing neuroinflammation (Garcia-Gutierrez et al., 2020; Srikantha and Mohajeri, 2019). TNF-ɑ is also thought to act directly on the CNS to cause the release of corticotropin-releasing factor (CRF), an anorexigenic neuropeptide that also affects gastric emptying, immune response, and mood (Holden and Pakula, 1996; Furman, 2007). Moreover, elevated TNF-ɑ results in hypothalamic dysregulation manifesting in reduced insulin and increased cortisol, which further decreases IL-1, IL-2, and 5-HT secretion, and thereby impacts serotonergic signaling (Holden and Pakula, 1996).
Underrepresentation of Bifidobacterium as a mechanism of ASD through decreased SCFAs
Bifidobacterium has been repeatedly measured in decreased quantity in the microbiomes of ASD patients (Xu et al., 2019; Oleskin et al., 2016; Fattorusso et al., 2019; Azhari et al., 2018; Garcia-Gutierrez et al., 2020). Given that Bifidobacterium is a producer of SCFAs, low Bifidobacterium in ASD patients might explain decreased SCFAs and dysregulation of the gut mucosal barrier, anti-inflammatory pathways, vagal signaling, appetite, serotonin production, neurodevelopment, and gene expression (Xu et al., 2019; Mangiola et al., 2016; Dempsey et al., 2019; Garcia-Gutierrez et al., 2020; Yang et al., 2019; Nankova et al., 2014; Oleskin et al., 2016; van de Wouw et al., 2017; Fields et al., 2018; Srikantha and Mohajeri, 2019). It is thought that adequate amounts of metabolites from Bifidobacterium in particular are essential for the regulation of the hypothalamus-pituitary-adrenal (HPA) axis; thus, decreased Bifidobacterium levels may contribute to impaired stress response and explain elevated cortisol levels in ASD fecal samples (Oleskin et al., 2016; Rudzki and Szulc, 2018; Obrenovich, 2018). Furthermore, an altered stress response would perpetuate immune dysregulation, contributing to neuroinflammation, GBB and BBB permeability, and autoimmune comorbidities.
Underrepresentation of Prevotella as a mechanism of ASD
Finally, it is worth briefly noting decreased levels of Prevotella in ASD, given the bacterium is negatively associated with gut permeability and positively associated with GI dysfunction (Tye et al., 2018; Fattorusso et al., 2019; Fields et al., 2018; Garcia-Gutierrez et al., 2020). Low Prevotella is also associated with increased leptin and decreased ghrelin, possibly accounting for feeding-related comorbidities in ASD (Seitz et al., 2019).
MGB axis dysfunction as a mechanism of AN pathogenesis
Anorexia nervosa (AN) is another neurological disorder which has recently received attention as a condition possibly linked to MGB axis dysfunction. With MGB axis-related traits and comorbidities such as gut dysbiosis, mood disorders, leaky gut, GI dysfunction, autoimmune disorders, sensory processing disorders, alexithymia, abnormal amygdala activity, impaired social functioning, serotonergic dysregulation, OCD, and ASD, AN is plausibly perpetuated by the immunological, nervous, and endocrine effects of gut dysbiosis (Hommer and Swedo, 2021; Gibson and Mehler, 2019; Bentz et al., 2016; Kerbeshian and Burd, 2009; van de Wouw et al., 2017; Galiana-Simal et al., 2017; Joos et al., 2011; Holden and Pakula, 1996; Karakula-Juchnowicz et al., 2017). Moreover, as a psychiatric disease dealing expressly with gut-related activities (such as eating), it makes sense to consider AN in relation to gut-brain communication. It is thought that interactions between the ENS and microbiota are involved in regulating AN-related processes such as food choice-related emotions, food intake, dopaminergic signaling, satiety, and hunger (Oleskin et al., 2016).
Once again, it is important to consider that many of the traits listed above (including dysbiosis and leaky gut) may be the result (and not the cause) of AN behavior, psychological or physiological stress, and/or other possible etiological pathways, and that a causal relationship between gut dysbiosis and AN has yet to be established (Breton et al., 2021; Gibson and Mehler, 2019; Glenny et al., 2017; Karakula-Juchnowicz et al., 2017; Liang et al., 2018). Repeated observations of dysbiosis in AN patients and existing knowledge linking MGB axis-related factors to AN symptomatology suggest that further investigation into a causal relationship between gut dysbiosis and AN is warranted (MacFabe, 2012; Breton et al., 2021; Gibson and Mehler, 2019; Seitz et al., 2019; van de Wouw et al., 2017; Glenny et al., 2017; Holden and Pakula, 1996; Karakula-Juchnowicz et al., 2017; Liang et al., 2018). Additionally, experiments modifying the microbiomes of mice have demonstrated a causal link between microbiome composition and anxious and depressive behaviors (Roubalovà et al., 2020). Finally, clinically noted overlaps between AN and ASD, paired with the earlier discussed evidence for a role of the MGB axis in the etiology of some forms of ASD, further support the consideration of AN as a MGB axis-related disorder (Kerbeshian and Burd, 2009; Galiana-Simal et al., 2017; Dattaro 2020).
Applying the gut-immune-brain paradigm to AN
Similar to Azhari and colleagues’ gut-immune-brain paradigm of ASD, an etiological pattern of AN can be derived from various MGB axis-related theories of neurological disorder in the literature (2017). Several sources propose gut dysbiosis as a causal factor of AN behaviors (van de Wouw et al., 2017; Karakula-Juchnowicz et al., 2017; Breton et al., 2021; Seitz et al., 2019). Both Karakula-Juchnowicz and colleagues and Seitz and colleagues hypothesize gut dysbiosis as a likely mechanism of AN pathogenesis through the induction of intestinal permeability and resulting immune response which ultimately interferes with mood and appetite (2017; 2019). This is supported by reports of a higher chance of autoimmune disorders in patients with AN (Seitz et al., 2019; Hommer and Swedo, 2017). While dysbiosis could occur for many reasons, it is known that malnutrition (for instance, as a result of restrictive feeding behaviors) alters the microbiome—for example, by promoting bacteria suited to a low-energy environment (Karakula-Juchnowicz et al., 2017; Glenny et al., 2017; Breton et al., 2021). In line with the prevailing thought that stress anticipates restrictive episodes, it is also known that stress alters the microbiome (Liu and Zhu, 2018; Rudzki and Szulc, 2018; Liang et al., 2018). It has been otherwise suggested that AN-related dysbiosis and GI permeability can be the result of infection and/or antibiotic use, which have been noted to commonly precede the onset of restrictive behaviors (Liang et al., 2018; Rudzki and Szulc, 2018; Holden and Pakula, 1996; Fetissov and Hökfelt, 2019). For example, it appears that Salmonella exposure may prompt overgrowth of the Enterobacteriaceae which are known to produce the ClpB mimetic of ɑ-melanocyte-stimulating hormone (ɑ-MSH), inducing satiety and behavior common to AN (Fetissov and Hökfelt, 2019).
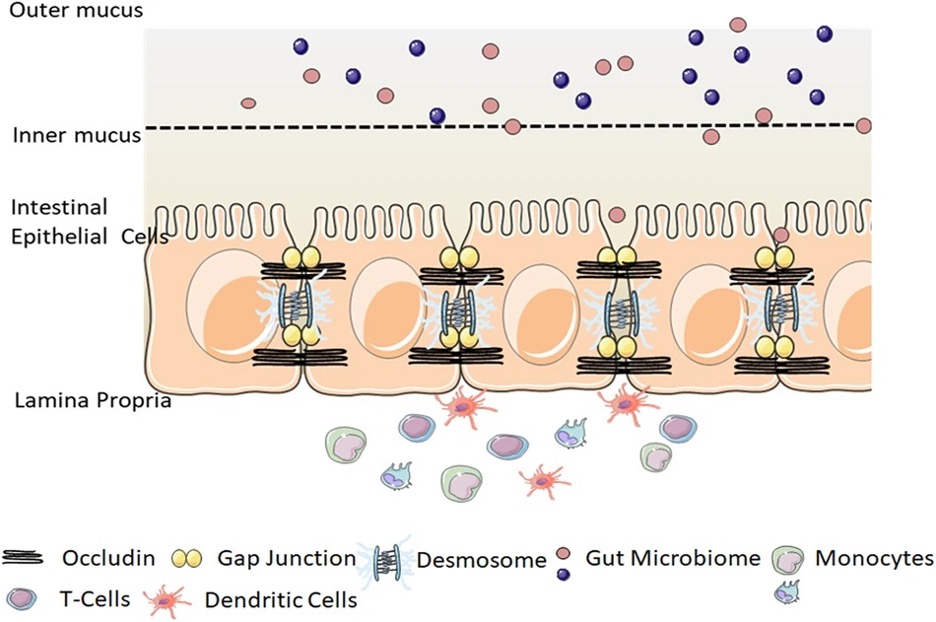
Figure 4: The gut-blood barrier (GBB). Dysbiosis appears to compromise the integrity of the intestinal mucosal barrier through a variety of mechanisms, allowing for the circulation of bacterial products in the bloodstream
Source: Wikimedia Commons
Regardless of the cause of dysbiosis, it is thought to cause an altered stress response and a leaky gut (Seitz et al., 2019; Karakula-Juchnowicz et al., 2017). Increased intestinal permeability has been reported in murine models of AN, and weight loss has been reported to increase intestinal permeability, although whether this is the result of gut dysbiosis or another mechanism is still unclear (Gibson and Mehler, 2019; Karakula-Juchnowicz et al., 2017). Leaky gut allows LPS to enter the bloodstream and incite systemic and neurological inflammation, creating a feedback loop through which gut dysbiosis, permeability, and inflammation are exacerbated, manifesting in the worsening symptoms of AN through mood dysregulation and immune-related appetite depletion (Karakula-Juchnowicz et al., 2017). It has been proposed that immune dysfunction leads to abnormal appetite, emotion, and weight regulation specifically through the production of autoantibodies against appetite and emotion regulating neuropeptides such as melanocortin, ɑ-MSH, and neuropeptide Y (NPY) (Fetissov et al., 2008).
Elevated TNF-ɑ in AN
Physical wasting in AN patients is additionally associated with elevated TNF-ɑ, and while a causal relationship has yet to be determined between these two events, it is known that TNF-ɑ acts on tight junctions to further increase GBB and BBB permeability, perhaps thereby leading to and/or worsening AN symptomatology (Karakula-Juchnowicz et al., 2017). Holden and Pakula similarly point to TNF-ɑ and immune system dysregulation as the underlying mechanism of AN, as an overexpression of TNF-ɑ leads to reduced β-endorphin and IL-4 levels, which further increases TNF-ɑ; this positive feedback loop could explain the difficulty of terminating gradually worsening behaviors in AN (1996). Furthermore, TNF-ɑ is thought to induce release of the anorexigenic neuropeptide CRF, reduce insulin, IL-1, and IL-2, and increase cortisol, thereby inhibiting 5-HT release and impairing serotonergic signaling, a known pathophysiological trait in AN (Holden and Pakula, 1996).
Altered SCFA levels in AN
Gut dysbiosis may further contribute to AN symptoms through decreased SCFA levels (Dempsey et al., 2019; MacFabe 2012; Gibson and Mehler, 2019; van de Wouw et al., 2017; Lin et al., 2012). Similar to the pathophysiology in ASD, properly balanced SCFA levels seem to be essential for processes also related to AN such as neurodevelopment, social behavior, serotonergic signaling, HPA stress response, GBB and BBB integrity, gastric motility, immune function, appetite and weight regulation, repetitive behaviors, and amygdala activity (Dempsey et al., 2019; Garcia-Gutierrez et al., 2020; Lobzhanidze et al., 2019; Yang et al., 2019; Nankova et al., 2014; MacFabe 2012; Oleskin et al., 2016; Valles-Colomer et al., 2019; van de Wouw et al., 2017; Lin et al., 2012). In particular, SCFAs regulate gut-derived neuropeptide signaling relating to appetite and metabolism (Oleskin et al., 2016; van de Wouw et al., 2017; Fetissov et al., 2008). Additionally, the effects of abnormal SCFA production as a result of gut dysbiosis may be compounded by the disruption of bacterial cross-feeding in the microbiome, and SCFAs may also contribute to AN by epigenetic gene modification (van de Wouw et al., 2017; Nankova et al., 2014). Furthermore, because SCFAs regulate inflammatory immune pathways, altered SCFA levels may perpetuate a vicious cycle of inflammation, dysbiosis, abnormal metabolite circulation, inflammation, and so on (van de Wouw et al., 2017). As SCFA-related inflammation can lead to gut permeability, further altering SCFA levels in serum, the cycle continues (Fields et al., 2018). The exact effects of high versus low levels of SCFAs on AN-related processes are unclear, but the data demonstrate that abnormal SCFA levels on either end of the spectrum have detrimental effects, and further investigation is warranted (van de Wouw et al., 2017).
Patterns of dysbiosis in AN
Given the prevalence of dysbiosis in AN cases, it may be helpful to synthesize patterns of dysbiosis in the literature and analyze their potential impacts on AN symptomatology. Faecalibacterium, Methanobrevibacter, and Actinobacteria have been reported in excess in individuals with AN, while Clostridium, Bacteroidetes, Prevotella, Roseburia, and overall bacterial diversity are reduced (Azhari et al., 2018; Dempsey et al., 2019; Glenny et al., 2017; Karakula-Juchnowicz et al., 2017; Breton et al., 2021; van de Wouw et al., 2017; Tye et al., 2018; Seitz et al., 2019; Roubalovà et al., 2020). However, there are studies contradicting some of these findings (Breton et al., 2021). A very recent systematic review reports increased Enterobacteriaceae, Parabacteroidetes, Alistipes, and Akkermansia, and decreased butyrate-producing bacteria such as Roseburia in AN (Lodovico et al., 2021). The lack of consensus on a microbiome profile, in AN as well as ASD and even healthy persons, should be noted and perhaps indicates that if there exists a causal relationship between dysbiosis and these disorders, it may not be due to the over- or undergrowth of one or another species but rather a complex system of dependent relationships. Alternatively, the heterogeneity of methodology in measuring microbiome composition could account for the lack of consensus (Lodovico et al., 2021).
MGB axis dysfunction as an explanation for the overlap between ASD and AN
ASD and AN share a high and thus far unexplained comorbidity rate which may be accounted for by a common etiological mechanism rooted in MGB axis dysfunction. People with AN are over 15 times more likely to be autistic than people without the condition, and people with ASD are over five times more likely to have AN (Dattaro, 2020). Within the eating disorder population at large, ASD is overrepresented at about 22.9% (compared to 1 in 54, or 1.85%, in the general population), and a standardized clinical assessment confirmed these findings within a cohort of AN patients, with 23.3% qualifying for ASD (Westwood et al., 2017). Such a remarkable comorbidity rate surely hints at shared or similar pathophysiological mechanisms. While AN is typically thought of as a purely psychiatric disease, some scientists propose it is better categorized as a neuropsychiatric developmental disorder, given the overlap of comorbidities between AN and ASD (Kerbeshian and Burd, 2009). In addition to the AN-ASD overlap, the two conditions share many other comorbidities which could be explained by the gut-immune-brain paradigm.
GI dysfunction in both ASD and AN
In both AN and ASD, GI dysfunction is common, particularly presenting in the form of constipation (Karakula-Juchnowicz et al., 2017; Garcia-Gutierrez et al., 2020; Glenny et al., 2017; Israelyan and Margolis, 2019; Sanctuary et al., 2018). In AN, it is thought that constipation is the result of the body slowing down digestive processes to optimize nutrient absorption during malnutrition and may be related to gut dysbiosis; increased levels of methanogenic bacteria species have been observed in correlation with constipation and are thought to be associated with nutrient absorption (Karakula-Juchnowicz et al., 2017; Seitz et al., 2019; Glenny et al. 2017). While constipation does not present in all ASD patients and occurs in the absence of a restrictive diet, it is linked to autoimmune dysfunction, anxiety, and sensory processing, suggesting that the MGB axis plays a role (Garcia-Gutierrez et al., 2020; Tye et al., 2018). Thus, the heterogeneity of GI symptoms in ASD patients may be explained by a shared MGB axis-related pathophysiology between AN and an immune-mediated subtype of ASD.
More specifically, GI dysfunction in ASD correlates with elevated PPA and TNF-ɑ; the relevance of these molecules to AN etiology as well suggests these pathways may explain the symptomatic overlap between the two conditions (Nankova et al., 2014; Srikantha and Mohajeri, 2019). It is also possible that the two mechanisms are entirely different and independent; however, given correlations between GI dysfunction and other ASD and AN symptoms such as social impairment and food selectivity, it seems possible that the mechanisms for GI dysfunction are shared, and further research should be conducted on this connection.
Explaining shared behavioral comorbidities in ASD and AN
Impaired social functioning
While impaired social functioning is better known as a hallmark trait of ASD, it appears to be a stable trait of AN as well (Israelyan and Margolis, 2019; Bentz et al., 2016; Kerr-Gaffney et al., 2020). Given that social impairment and associated brain abnormalities also tend to correlate with length of AN episodes, it seems plausible that MGB axis-directed neurodevelopment is disrupted in AN, contributing to an ASD-like phenotype; it is also notable that AN commonly appears during the teenage years, which are thought to be a critical window for development of social perception skills and the development of the amygdala (Bentz et al., 2016; Lobzhanidze et al., 2019).
In ASD, social impairment is correlated with GI dysfunction (particularly constipation), leading researchers to think that simultaneous developmental defects in the brain, intestine, and/or MGB axis underlie ASD pathogenesis (Israelyan and Margolis, 2019). Meanwhile, children with social difficulties are more likely to develop disordered eating; it is hypothesized that such children with ASD or ASD-like traits engage in starvation as a coping mechanism to numb difficult or mal-processed emotions, which could then lead to developmental brain abnormalities exacerbating ASD symptoms (Dattaro, 2020). Regardless of causality, the shared trait of abnormal social functioning has been linked to gut dysbiosis, altered PPA levels, increased GI serotonin secretion, and amygdala hypoactivity and structural abnormalities (Liang et al., 2018; Garcia-Gutierrez et al., 2020; MacFabe, 2012; Herrington et al., 2016; Lobzhanidze et al., 2019; Nankova et al., 2014). Since dysbiosis-induced altered PPA levels are associated with social impairment in addition to abnormal serotonergic signaling, neurodevelopment, and amygdala structure and function, it seems possible that PPA is behind the link between social difficulties in both AN and an immune-mediated subtype of ASD (Dempsey et al., 2019; Garcia-Gutierrez et al., 2020; Yang et al., 2019; van de Wouw et al., 2017; Nankova et al., 2014; Lobzhanidze et al., 2019; MacFabe 2012). That being said, the fact that PPA has been measured in excess in ASD and in deficit in AN must be considered (Dempsey et al., 2019; Srikantha and Mohajeri, 2019; Shultz et al., 2014; Garcia-Gutierrez et al., 2020; Nankova et al., 2014). The data on the effects of PPA and other SCFAs on behavior and physiology are conflicting, with both elevated and decreased levels showing similar detrimental effects (van de Wouw et al., 2017). It is possible that SCFA signaling is dependent on mere balance, rather than remaining within a specific level.
Impaired emotional processing and alexithymia
Both ASD and AN patients also tend to display impaired emotional processing and alexithymia, the inability to name one’s own or others’ emotions (Lobzhanidze et al., 2019; Bentz et al., 2016; Holden and Pakula, 1996; Kerr-Gaffney et al., 2020). Once again, PPA levels seem to be connected to these symptoms, as they are abnormal in both ASD and AN individuals, and emotional processing is in part regulated by the amygdala, which is affected by PPA (Lobzhanidze et al., 2019; Dempsey et al., 2019; Shultz et al., 2014; Garcia-Gutierrez et al., 2020; Srikantha and Mohajeri, 2019; Nankova et al., 2014; MacFabe, 2012). Additionally, amygdala activity is also regulated by LPS (Mangiola et al., 2016). Thus, the LPS/TNF-ɑ overproduction in AN and ASD seems to form another piece of the puzzle which may also be worth further investigation (Srikantha and Mohajeri, 2019; Rudzki and Szulc, 2018; Garcia-Gutierrez et al., 2020; van de Wouw et al., 2017; Fields et al., 2018; Karakula-Juchnowicz et al., 2017; Mangiola et al., 2016; Xu et al., 2019; Holden and Pakula, 1996; Azhari et al., 2018).
Obsessive/compulsive and repetitive behaviors
Furthermore, ASD and AN individuals are both known for obsessive/compulsive and repetitive behaviors, manifesting in tics in the former and aberrant feeding behaviors in the latter. This also seems to be connected with abnormal PPA levels: altered PPA and serotonin levels are associated with obsessive/compulsive and repetitive behaviors, and serotonin signaling is regulated in part by PPA (Dempsey et al., 2019; MacFabe 2012; Nankova et al., 2014; Garcia-Gutierez et al., 2020). LPS, which is elevated in ASD and AN, is also associated with repetitive behaviors and anxiety in mice (Fields et al., 2018; Xu et al., 2019; Srikantha and Mohajeri, 2019; Rudzki and Szulc, 2018; Mangiola et al., 2016; Garcia-Gutierrez et al., 2020; van de Wouw et al., 2017; Karakula-Juchnowicz et al., 2017; Azhari et al., 2018).
Impaired sensory processing and stress response
ASD and AN patients also may exhibit sensory hypersensitivity and anxiety disorders (Herrington et al., 2016; Galiana-Simal et al., 2017; Dattaro, 2020; Garcia-Gutierrez et al., 2020; Tye et al., 2018; Fields et al., 2018; Lobzhanidze et al., 2019). In fact, sensory processing disorder (SPD) presents in about 90% of ASD cases, and it has been suggested that a subset of AN cases are “sensory eating disorders” involving hyperreactivity to certain food traits; the fear of weight gain common in individuals with AN might be linked to hypersensitivity to the sensations of weight gain (Galiana-Simal et al., 2017). Moreover, recovered AN patients show impaired perception of social stimulation, which might be tied to abnormal sensory processing (Bentz et al., 2016). In ASD, sensory processing, anxiety, and GI symptoms are linked, and the appearance of these comorbidities in AN as well suggests a MGB axis-related pathophysiology (Tye et al., 2018). Indeed, it is thought that the microbiota is implicated in sensory-motor processing and filtering (Fields et al., 2018). This is supported by encouraging results from studies showing the potential for probiotics to ameliorate sensory responsiveness in ASD (Garcia-Gutierrez et al., 2020).
Underlying the connection between anxiety and the microbiota appears to be abnormal amygdala habituation, altered PPA/SCFA levels, abnormal HPA axis development, altered autoantibodies against mood and appetite-regulating neuropeptides, and elevated intestinal LPS (Herrington et al., 2016; Lobzhanidze et al., 2016; MacFabe, 2012; Garcia-Gutierrez et al., 2020; Oleskin et al., 2016; Liang et al., 2018; Glenny et al., 2017; Fetissov et al., 2008; Xu et al., 2019). However, one study showed no improvement in anxiety in mice with PPA regulation (Lobzhanidze et al., 2019). It is thought that certain anxiety disorders such as panic disorder occur at higher rates in the ASD population, and panic disorder might be related to sensory hypersensitivity (Herrington et al., 2016). Thus, there seems to be a link between sensory hyper-responsiveness, anxiety disorders, and the MGB axis in ASD and AN driven by PPA/SCFA and/or LPS levels.
Abnormal feeding behaviors
Finally, while AN is best known for inappetence and reduced food intake, these symptoms (alongside other feeding abnormalities) are also prevalent in the ASD population; as mentioned earlier, children with social difficulties are more likely to develop disordered eating (Vissoker et al., 2015; Dattaro, 2020). Within the ASD population, food selectivity is correlated with GI dysfunction and is thought to be caused by discomfort, possibly relating to sensory hypersensitivity to foods and/or GI sensations (Vissoker et al., 2015; Galiana-Simal et al., 2017). Yet, it is also possible that there is a deeper mechanism at work. Multiple sources report that the MGB axis plays an important role in food choice, appetite, hunger, and satiety, as well as affecting the emotions regarding these factors (Oleskin et al., 2016; van de Wouw et al., 2017; Liang et al., 2018). Broadly, there is a correlation between dysbiosis and inappetence (Seitz et al., 2019). Specifically, SCFAs affect neuropeptides and gut hormones regulating appetite, intake, metabolism, and emotions regarding food choice (Oleskin et al., 2016; Fetissov et al., 2008; Lin et al., 2012; Lobzhanidze et al., 2019; MacFabe, 2012; Dempsey et al., 2019; Garcia-Gutierrez et al., 2020; van de Wouw et al., 2017; Holden and Pakula, 1996). PPA and BA have been shown to reduce intake by stimulating secretion of anorexigenic hormones PYY and GLP-1 via the vagus nerve; NPY and PYY are involved in appetite and are associated with AN and indigestion (Lin et al., 2012; Lobzhanidze et al., 2019; MacFabe, 2012; Dempsey et al., 2019; Garcia-Gutierrez et al., 2020; van de Wouw et al., 2017; Holden and Pakula, 1996). Increased PPA could explain disordered eating in ASD, but the lack of PPA in AN presents a challenge to the SCFA appetite-regulation hypothesis. However, as noted before, there are contradicting data on the effects of high versus low SCFAs on physiological processes; for example, PPA has been shown to either increase or decrease metabolism and appetite (van de Wouw et al., 2017). Future research should focus on a better understanding of SCFAs’ involvement in feeding behavior.
Another hypothesis for the regulation of intake focuses on dysbiosis-related immune-mediated processes. Immune dysfunction is correlated with decreased appetite, and more precisely, infection via the vagus nerve induces inappetence (Karakula-Juchnowicz et al., 2018; Liang et al., 2018). In AN, bacterially induced cross-reactive autoantibodies against ɑ-MSH are linked to anxiety and are thought to produce inappetence through molecular mimicry, activating the appetite-regulating melanocortin complex (Aresti Sanz and El Aidy, 2019; Karakula-Juchnowicz et al., 2017; van de Wouw et al., 2017; Glenny et al., 2017; Fetissov and Hökfelt, 2019). It is thought that production of these cross-reactive antibodies is stimulated by ClpB production in the presence of E. coli overgrowth, and the chronic stimulation of melanocortin type 4 receptor has been pharmacologically shown to induce anorexia and weight loss (Fetissov and Hökfelt, 2019; Karakula-Juchnowicz et al., 2017). While neither of these has been noted in ASD, it may be worth acknowledging high observed levels of Proteobacteria, under which E. coli is categorized, in ASD (Azhari et al., 2018; Fattorusso et al., 2019; Tye et al., 2018; Fields et al., 2018). This perhaps signals a mechanism similar to the autoantibody molecular mimicry in AN. Between the SCFA-neuropeptide hypothesis and the molecular mimicry hypothesis, it is plausible that gut dysbiosis may contribute to inappetence and reduced intake in both ASD and AN, and further research should be concentrated on this question to better understand the shared pathophysiology.
Conclusion: Altered SCFA levels, LPS and TNF-ɑ as potential explanations for ASD and AN comorbidities
Both ASD and AN are neurological disorders commonly featuring GI and immune dysfunction. As the nexus between the largest immune organ (the GI tract) and the CNS, the MGB axis has the power to explain the connection between GI, autoimmune, and behavioral comorbidities in ASD and AN, as well as co-occurrences of the two disorders themselves (Rudzki and Szulc, 2018). With both ASD and AN individuals known to present with dysbiosis, scientists have recently investigated the role of the microbiota – one end of the MGB axis – as a diagnostic and therapeutic tool in disorders commonly diagnosed and treated exclusively at the brain – the other end of the axis.
In order to develop practical microbiological applications for preventing and treating neurological disorders, it is essential to first understand how changes to the microbiome and the MGB axis contribute to such conditions. For example, by studying ASD and AN from the perspective of the MGB axis, key overlaps in physiological traits could explain symptoms in both disorders independently and in conjunction. Most notably, SCFA, LPS, and TNF-ɑ are all linked to the MGB axis and have been repeatedly noted in abnormal quantities in both ASD and AN. Altered SCFAs are associated with impaired GI functioning, social functioning, emotional processing, sensory processing, and stress response, all of which are found in ASD and AN. Elevated levels of TNF-ɑ in serum correlate with impaired GI function and emotional processing, while elevated LPS correlates with impaired emotional processing, sensory processing, and stress response.

Figure 5: Mice can be used as models of AN and ASD when testing the effects of MGB axis-related molecules such as SCFAs, LPS, and TNF-ɑ; murine modeling has also demonstrated the potential for probiotic and FMT treatment of neurological disorders. The next step is to conduct high-power human studies for such novel treatments
Source: Wikimedia Commons
While these correlations do not indicate causality, murine modeling has demonstrated a causal relationship between many of these factors, including between LPS and anxiety, repetitive behaviors, and neuroinflammation; SCFAs and BBB integrity; BA and anorexigenic hormones; PPA and neuroinflammation, anorexigenic hormones, and decreased social motivation; anorexigenic hormones (PYY) and constipation; and TNF-ɑ and intestinal permeability (Rudzki and Szulc, 2018; van de Wouw et al., 2017; Glenny et al., 2017; Lin et al., 2012; Lobzhanidze et al., 2019; Karakula-Juchnowicz et al., 2017). Certainly, it will be worthwhile to further investigate these connections by conducting high-power human studies. So far, probiotic and fecal transplantation studies have demonstrated an ability for microbiome remediation to alleviate GI and behavior symptoms in ASD (Mangiola et al., 2016; Srikantha and Mohajeri, 2019; Israelyan and Margolis, 2019). The current data on the causality between dysbiosis and human pathopsychology is nascent, and expanding such research should be prioritized in the coming years.
References
Aresti Sanz, J., & El Aidy, S. (2019). Microbiota and gut neuropeptides: A dual action of antimicrobial activity and neuroimmune response. Psychopharmacology, 236(5), 1597–1609. https://doi.org/10.1007/s00213-019-05224-0
Azhari, A., Azizan, F., & Esposito, G. (2019). A systematic review of gut-immune-brain mechanisms in Autism Spectrum Disorder. Developmental Psychobiology, 61(5), 752–771. https://doi.org/10.1002/dev.21803
Bentz, M., Jepsen, J. R. M., Pedersen, T., Bulik, C. M., Pedersen, L., Pagsberg, A. K., & Plessen, K. J. (2017). Impairment of Social Function in Young Females With Recent-Onset Anorexia Nervosa and Recovered Individuals. Journal of Adolescent Health, 60(1), 23–32. https://doi.org/10.1016/j.jadohealth.2016.08.011
Breton, J., Tirelle, P., Hasanat, S., Pernot, A., L’Huillier, C., Rego, J.-C. do, Déchelotte, P., Coëffier, M., Bindels, L. B., & Ribet, D. (2021). Gut microbiota alteration in a mouse model of Anorexia Nervosa. Clinical Nutrition, 40(1), 181–189. https://doi.org/10.1016/j.clnu.2020.05.002
CDC. (2020, September 25). Data and Statistics on Autism Spectrum Disorder | CDC. Centers for Disease Control and Prevention. https://www.cdc.gov/ncbddd/autism/data.html
Cerdó, T., Diéguez, E., & Campoy, C. (2019). Early nutrition and gut microbiome: Interrelationship between bacterial metabolism, immune system, brain structure, and neurodevelopment. American Journal of Physiology-Endocrinology and Metabolism, 317(4), E617–E630. https://doi.org/10.1152/ajpendo.00188.2019
Dattaro, L. (2020, December 7). Anorexia’s link to autism, explained. Spectrum | Autism Research News. https://www.spectrumnews.org/news/anorexias-link-to-autism-explained/
Dempsey, J. L., Little, M., & Cui, J. Y. (2019). Gut microbiome: An intermediary to neurotoxicity. NeuroToxicology, 75, 41–69. https://doi.org/10.1016/j.neuro.2019.08.005
Di Lodovico, L., Mondot, S., Doré, J., Mack, I., Hanachi, M., & Gorwood, P. (2021). Anorexia nervosa and gut microbiota: A systematic review and quantitative synthesis of pooled microbiological data. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 106, 110114. https://doi.org/10.1016/j.pnpbp.2020.110114
Fattorusso, A., Di Genova, L., Dell’Isola, G. B., Mencaroni, E., & Esposito, S. (2019). Autism Spectrum Disorders and the Gut Microbiota. Nutrients, 11(3), 521. https://doi.org/10.3390/nu11030521
Fetissov, S. O., Hamze Sinno, M., Coquerel, Q., Do Rego, J. C., Coëffier, M., Gilbert, D., Hökfelt, T., & Déchelotte, P. (2008). Emerging role of autoantibodies against appetite-regulating neuropeptides in eating disorders. Nutrition, 24(9), 854–859. https://doi.org/10.1016/j.nut.2008.06.021
Fetissov, S. O., & Hökfelt, T. (2019). On the origin of eating disorders: Altered signaling between gut microbiota, adaptive immunity and the brain melanocortin system regulating feeding behavior. Current Opinion in Pharmacology, 48, 82–91. https://doi.org/10.1016/j.coph.2019.07.004
Fields, C. T., Sampson, T. R., Bruce-Keller, A. J., Kiraly, D. D., Hsiao, E. Y., & de Vries, G. J. (2018). Defining Dysbiosis in Disorders of Movement and Motivation. The Journal of Neuroscience, 38(44), 9414–9422. https://doi.org/10.1523/JNEUROSCI.1672-18.2018
Furman, B. L. (2007). Corticotropin Releasing Factor. In S. J. Enna & D. B. Bylund (Eds.), xPharm: The Comprehensive Pharmacology Reference (pp. 1–4). Elsevier. https://doi.org/10.1016/B978-008055232-3.61510-7
Galiana-Simal, A., Muñoz-Martinez, V., & Beato-Fernandez, L. (2017). Connecting Eating Disorders and Sensory Processing Disorder: A Sensory Eating Disorder Hypothesis. Global Journal of Intellectual and Developmental Disabilities.
Garcia-Gutierrez, E., Narbad, A., & Rodríguez, J. M. (2020). Autism Spectrum Disorder Associated With Gut Microbiota at Immune, Metabolomic, and Neuroactive Level. Frontiers in Neuroscience, 14. https://doi.org/10.3389/fnins.2020.578666
Gibson, D., & Mehler, P. S. (2019). Anorexia Nervosa and the Immune System—A Narrative Review. Journal of Clinical Medicine, 8(11). https://doi.org/10.3390/jcm8111915
Glenny, E. M., Bulik-Sullivan, E. C., Tang, Q., Bulik, C. M., & Carroll, I. M. (2017). Eating disorders and the intestinal microbiota: Mechanisms of energy homeostasis and behavioral influence. Current Psychiatry Reports, 19(8), 51. https://doi.org/10.1007/s11920-017-0797-3
Groves, H. T., Higham, S. L., Moffatt, M. F., Cox, M. J., & Tregoning, J. S. (2020). Respiratory Viral Infection Alters the Gut Microbiota by Inducing Inappetence. MBio, 11(1), e03236-19, /mbio/11/1/mBio.03236-19.atom. https://doi.org/10.1128/mBio.03236-19
Herrington, J. D., Miller, J. S., Pandey, J., & Schultz, R. T. (2016). Anxiety and social deficits have distinct relationships with amygdala function in autism spectrum disorder. Social Cognitive and Affective Neuroscience, 11(6), 907–914. https://doi.org/10.1093/scan/nsw015
Holden, R. J., & Pakula, I. S. (1996). The role of tumor necrosis factor-alpha in the pathogenesis of anorexia and bulimia nervosa, cancer cachexia and obesity. Medical Hypotheses, 47(6), 423–438. https://doi.org/10.1016/s0306-9877(96)90153-x
Hommer, R. E., & Swedo, S. E. (2017). Anorexia and Autoimmunity: Challenging the Etiologic Constructs of Disordered Eating. Pediatrics, 140(6), e20173060. https://doi.org/10.1542/peds.2017-3060
Israelyan, N., & Margolis, K. G. (2019). Reprint of: Serotonin as a link between the gut-brain-microbiome axis in autism spectrum disorders. Pharmacological Research, 140, 115–120. https://doi.org/10.1016/j.phrs.2018.12.023
Kang, D.-W., Adams, J. B., Gregory, A. C., Borody, T., Chittick, L., Fasano, A., Khoruts, A., Geis, E., Maldonado, J., McDonough-Means, S., Pollard, E. L., Roux, S., Sadowsky, M. J., Lipson, K. S., Sullivan, M. B., Caporaso, J. G., & Krajmalnik-Brown, R. (2017). Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome, 5(1). Scopus. https://doi.org/10.1186/s40168-016-0225-7
Karakuła-Juchnowicz, H., Pankowicz, H., Juchnowicz, D., Valverde Piedra, J., & Małecka-Massalska, T. (2017). INTESTINAL MICROBIOTA – A KEY TO UNDERSTANDING THE PATHOPHYSIOLOGY OF ANOREXIA NERVOSA? Psychiatria Polska, 51(5), 859–870. https://doi.org/10.12740/PP/65308
Kerbeshian, J., & Burd, L. (2009). Is anorexia nervosa a neuropsychiatric developmental disorder? An illustrative case report. The World Journal of Biological Psychiatry, 10(4–2), 648–657. https://doi.org/10.1080/15622970802043117
Kerr-Gaffney, J., Harrison, A., & Tchanturia, K. (2020). Autism spectrum disorder traits are associated with empathic abilities in adults with anorexia nervosa. Journal of Affective Disorders, 266, 273–281. https://doi.org/10.1016/j.jad.2020.01.169
Liang, S., Wu, X., & Jin, F. (2018). Gut-Brain Psychology: Rethinking Psychology From the Microbiota–Gut–Brain Axis. Frontiers in Integrative Neuroscience, 12. https://doi.org/10.3389/fnint.2018.00033
Lin, H. V., Frassetto, A., Kowalik Jr, E. J., Nawrocki, A. R., Lu, M. M., Kosinski, J. R., Hubert, J. A., Szeto, D., Yao, X., Forrest, G., & Marsh, D. J. (2012). Butyrate and Propionate Protect against Diet-Induced Obesity and Regulate Gut Hormones via Free Fatty Acid Receptor 3-Independent Mechanisms. PLoS ONE, 7(4). https://doi.org/10.1371/journal.pone.0035240
Liu, L., & Zhu, G. (2018). Gut–Brain Axis and Mood Disorder. Frontiers in Psychiatry, 9. https://doi.org/10.3389/fpsyt.2018.00223
Lobzhanidze, G., Lordkipanidze, T., Zhvania, M., Japaridze, N., MacFabe, D. F., Pochkidze, N., Gasimov, E., & Rzaev, F. (2019). Effect of propionic acid on the morphology of the amygdala in adolescent male rats and their behavior. Micron, 125, 102732. https://doi.org/10.1016/j.micron.2019.102732
MacFabe, D. F. (2012). Short-chain fatty acid fermentation products of the gut microbiome: Implications in autism spectrum disorders. Microbial Ecology in Health and Disease, 23. https://doi.org/10.3402/mehd.v23i0.19260
Mangiola, F., Ianiro, G., Franceschi, F., Fagiuoli, S., Gasbarrini, G., & Gasbarrini, A. (2016). Gut microbiota in autism and mood disorders. World Journal of Gastroenterology, 22(1), 361–368. https://doi.org/10.3748/wjg.v22.i1.361
Mayer, E. A. (2011). Gut feelings: The emerging biology of gut–brain communication. Nature Reviews Neuroscience, 12(8), 453–466. https://doi.org/10.1038/nrn3071
Obrenovich, M. E. M. (2018). Leaky Gut, Leaky Brain? Microorganisms, 6(4). https://doi.org/10.3390/microorganisms6040107
Oleskin, A. V., El’-Registan, G. I., & Shenderov, B. A. (2016). Role of neuromediators in the functioning of the human microbiota: “Business talks” among microorganisms and the microbiota-host dialogue. Microbiology, 85(1), 1–22. https://doi.org/10.1134/S0026261716010082
Pearson-Leary, J., Zhao, C., Bittinger, K., Eacret, D., Luz, S., Vigderman, A. S., Dayanim, G., & Bhatnagar, S. (2020). The gut microbiome regulates the increases in depressive-type behaviors and in inflammatory processes in the ventral hippocampus of stress vulnerable rats. Molecular Psychiatry, 25(5), 1068–1079. https://doi.org/10.1038/s41380-019-0380-x
Roubalová, R., Procházková, P., Papežová, H., Smitka, K., Bilej, M., & Tlaskalová-Hogenová, H. (2020). Anorexia nervosa: Gut microbiota-immune-brain interactions. Clinical Nutrition, 39(3), 676–684. https://doi.org/10.1016/j.clnu.2019.03.023
Rudzki, L., & Szulc, A. (2018). “Immune Gate” of Psychopathology—The Role of Gut Derived Immune Activation in Major Psychiatric Disorders. Frontiers in Psychiatry, 9. https://doi.org/10.3389/fpsyt.2018.00205
Sanctuary, M. R., Kain, J. N., Angkustsiri, K., & German, J. B. (2018). Dietary Considerations in Autism Spectrum Disorders: The Potential Role of Protein Digestion and Microbial Putrefaction in the Gut-Brain Axis. Frontiers in Nutrition, 5. https://doi.org/10.3389/fnut.2018.00040
Seitz, J., Belheouane, M., Schulz, N., Dempfle, A., Baines, J. F., & Herpertz-Dahlmann, B. (2019). The Impact of Starvation on the Microbiome and Gut-Brain Interaction in Anorexia Nervosa. Frontiers in Endocrinology, 10. https://doi.org/10.3389/fendo.2019.00041
Seitz, J., Trinh, S., & Herpertz-Dahlmann, B. (2019). The Microbiome and Eating Disorders- ClinicalKey. Clinical Key. https://www-clinicalkey-com.dartmouth.idm.oclc.org/#!/content/playContent/1-s2.0-S0193953X18311511?returnurl=null&referrer=null
Shultz, S. R., & MacFabe, D. F. (2014). Propionic Acid Animal Model of Autism. In V. B. Patel, V. R. Preedy, & C. R. Martin (Eds.), Comprehensive Guide to Autism (pp. 1755–1778). Springer. https://doi.org/10.1007/978-1-4614-4788-7_106
Srikantha, P., & Mohajeri, M. H. (2019). The Possible Role of the Microbiota-Gut-Brain-Axis in Autism Spectrum Disorder. International Journal of Molecular Sciences, 20(9), 2115. https://doi.org/10.3390/ijms20092115
Tye, C., Runicles, A. K., Whitehouse, A. J. O., & Alvares, G. A. (2019). Characterizing the Interplay Between Autism Spectrum Disorder and Comorbid Medical Conditions: An Integrative Review. Frontiers in Psychiatry, 9. https://doi.org/10.3389/fpsyt.2018.00751
Valles-Colomer, M., Falony, G., Darzi, Y., Tigchelaar, E. F., Wang, J., Tito, R. Y., Schiweck, C., Kurilshikov, A., Joossens, M., Wijmenga, C., Claes, S., Van Oudenhove, L., Zhernakova, A., Vieira-Silva, S., & Raes, J. (2019). The neuroactive potential of the human gut microbiota in quality of life and depression. Nature Microbiology, 4(4), 623–632. https://doi.org/10.1038/s41564-018-0337-x
van de Wouw, M., Schellekens, H., Dinan, T. G., & Cryan, J. F. (2017). Microbiota-Gut-Brain Axis: Modulator of Host Metabolism and Appetite. The Journal of Nutrition, 147(5), 727–745. https://doi.org/10.3945/jn.116.240481
Vissoker, R. E. (2015). Eating and feeding problems and gastrointestinal dysfunction in Autism Spectrum Disorders. Research in Autism Spectrum Disorders, 12.
Wang, M., Wan, J., Rong, H., He, F., Wang, H., Zhou, J., Cai, C., Wang, Y., Xu, R., Yin, Z., & Zhou, W. (2019). Alterations in Gut Glutamate Metabolism Associated with Changes in Gut Microbiota Composition in Children with Autism Spectrum Disorder. MSystems, 4(1). https://doi.org/10.1128/mSystems.00321-18
Werling, D. M., & Geschwind, D. H. (2013). Sex differences in autism spectrum disorders. Current Opinion in Neurology, 26(2), 146–153. https://doi.org/10.1097/WCO.0b013e32835ee548
Westwood, H., Mandy, W., & Tchanturia, K. (2017). Clinical evaluation of autistic symptoms in women with anorexia nervosa. Molecular Autism, 8(1), 12. https://doi.org/10.1186/s13229-017-0128-x
Xu, M., Xu, X., Li, J., & Li, F. (2019). Association Between Gut Microbiota and Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Frontiers in Psychiatry, 10. https://doi.org/10.3389/fpsyt.2019.00473
Yang, L. L., Millischer, V., Rodin, S., MacFabe, D. F., Villaescusa, J. C., & Lavebratt, C. (2020). Enteric short-chain fatty acids promote proliferation of human neural progenitor cells. Journal of Neurochemistry, 154(6), e14928. https://doi.org/10.1111/jnc.14928
Related Posts
An Introduction to the Ebola Virus
Cover Image: Blood sample of a patient infected by the...
Read MorePreventing Peripheral Artery Disease in African Americans from Georgia: A Public Health Initiative
This publication is in proud partnership with Project UNITY’s Catalyst Academy 2024...
Read MoreUniversal Donors: A New Enzymatic Approach to Blood and Organ Matching
Figure: In a 2019 study, researchers used a microbial sample...
Read MoreJillian Troth