Abstract:
Cancer treatment has long been an engaging field of study for researchers. Yet despite years of refinement, conventional treatment methods like radiotherapy and chemotherapy are still incompetent in the permanent cure of cancer. Recently, bacterial treatment approach has shown promising experimental results. Bacterial treatment allows for deeper tumor penetration than radiation due to the high mobility of bacterial cells. Their side effects are minimal if compared to chemo- and radiotherapy. Due to their oxygen sensitivity, anaerobic bacteria show incredible target specificity to tumors. This article reviews the current cutting-edge research in bacterial anticancer treatment with anaerobes, primarily focusing on novel strains of Salmonella, Clostridium and Escherichia coli. Later, we brief on various therapeutic payloads, synthetic biology techniques, and combination therapies being developed worldwide to aid bacterial treatment.
Keywords: Bacteria, Cancer, Anaerobes, Tumor Targeting
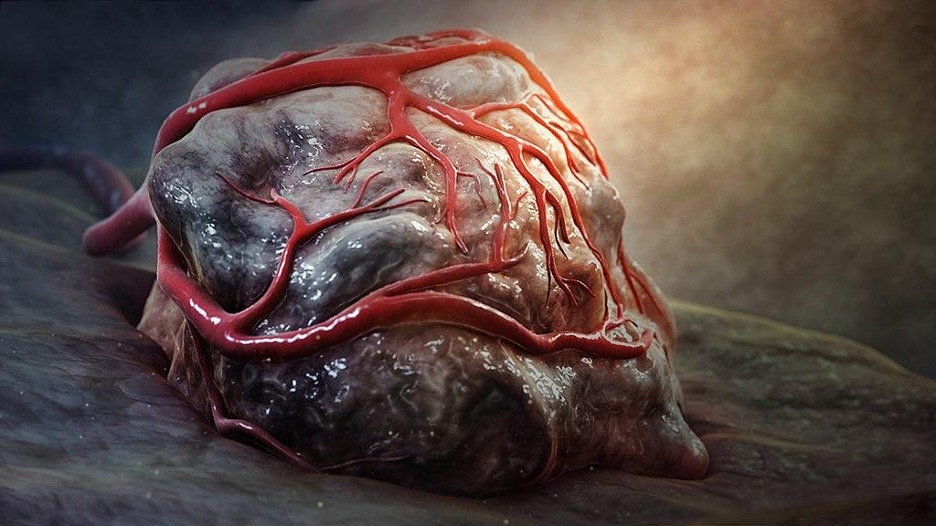
Figure: 3D still showing tumor. Researchers have engineered bacteria that can selectively colonize tumors and be used in anticancer treatments. (Source: Wikimedia Commons, Scientific Animations)
Introduction
Cancers, being one of the most widespread and deadliest diseases in the history of humanity, have long drawn the attention of medical professionals and researchers. Just in the year 2020, the World Health Organization (WHO) recorded 10 million deaths due to cancer (2022). Cancer arises from cells that lose the ability of ‘controlled division’ and become tumors. In later stages, these tumors become invasive and start proliferating in other parts of the body (malignant stage).
Conventional cancer treatments include combinations of surgery, chemotherapy, and radiotherapy as primary treatment, followed by adjuvant and palliative treatment. Radiation therapy is one of the earliest developed treatments for cancer, which uses high doses of radiation to kill cancer cells and shrink tumors. Radiation therapy has undergone significant improvements, but treating hypoxic cells (lacking an oxygenated environment) in malignant tumors is still challenging. Experiments show that hypoxic cells are up to three times more resistant to ionizing radiation than normal cells (Jiang et al., 2010). Other treatments are also not just incapable of permanently curing cancer, but also may produce numerous side effects such as multidrug resistance, diarrhea, and loss of appetite (Duong et al., 2019). Advancements like targeted therapy and immunotherapy are arising in the field of cancer therapeutics (Duong et al., 2019). However, even these novel approaches face challenges, including high toxicity to normal tissue and complications in treating deep cancers (Duong et al., 2019).
Administration of live tumor-targeting bacteria as a treatment has the potential to meet these challenges while harvesting better results than the conventional methods. Bacterial cancer treatment dates back to the 1890s when William Coley tried treating cancer with a mixture of killed bacteria, Streptococcus pyogenes and Serratia marcescens, popularly known as Coley’s toxin (Coley, 1936). Despite showing positive results, Coley’s toxin had a very high failure ratio. Since then, there have been many advancements in this field (“The Toxin Treatment Of Sarcoma,” 1908).
Bacteria can preferentially accumulate and proliferate within a tumor and initiate antitumor immune responses (Duong et al., 2019). Experiments with human xenografts in mice have already been very successful (Duong et al., 2019). Further programming with genetic manipulation and sophisticated synthetic bioengineering holds even more promising results (Duong et al., 2019). Unlike the passive distribution and limited penetration of intrinsic chemotherapeutic drugs, bacteria, being complex living organisms, offer unlimited transport capacity. They can be genetically programmed to self-propel and disperse themselves into tumor tissue. This ability enables bacteria to penetrate deeper into tumor tissue (Duong et al., 2019). In addition to intrinsic antitumor effects, bacteria also activate the immune cell population in the tumor microenvironment (TMEs) (Duong et al., 2019).
The TME possesses properties such as hypoxia, acidosis, and necrosis, which are conducive to the development of anaerobic bacteria and can be used to improve tumor specificity and targeting efficiency of BT (Bacterial treatment). Various bacterial strains of Escherichia coli, Salmonella, and Clostridium can be made to take advantage of such specificity and selectively colonize tumor regions. Their primary mechanisms may vary (Salmonella spp. induce apoptosis and/or autophagy in tumors, Clostridium spp. secretes bacterial toxins interfering with intracellular functions or perturbing cellular membranes, while E. coli can be genetically modified to release toxins or toxin activators which in turn kill tumor cells), but it is evident that they may offer a unique and efficient immunotherapy strategy (Duong et al., 2019).
Also, recent reports indicate that bacteria can target both primary (anatomically localized tumor, growing at the site where its progression began) and metastatic tumors (anatomically delocalized tumors, spread to other parts of the body). This feature is being exploited for tumor-selective drug delivery (Jiang et al., 2010). Moreover, to enhance the outcomes of bacterial cancer therapy, research is being conducted to combine it with eukaryotic and prokaryotic expression systems, cytotoxic agents, prodrug-converting enzymes, immune regulators, tumor stroma-targeting molecules, and siRNAs (Duong et al., 2019). Taking advantage of quorum sensing (QS) — a system which couples gene expression to the density of surrounding bacterial cells – bacteria can express genes toxic to cancer cells just when they proliferate in TMEs, where the ‘tumor to normal’ ratio (ratio between uptake tumor cells and uptake by surrounding tissues) acan exceed 10,000:1 (Duong et al., 2019). In this article, we explore the potential of various anaerobic bacteria in cancer treatment, as evidenced by experiments of various research groups worldwide (Duong et al., 2019).
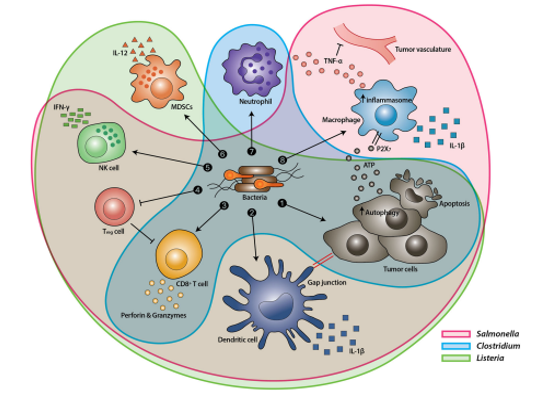
Figure 1: Tumor Targeting Mechanism of Various Bacteria. (Source: Bacteria-cancer interactions bacteria-based cancer therapy by Duong et al., 2019, CC BY 4.0)
Salmonella
Salmonella typhimurium is the most widely studied bacteria for BT. The bacterium is seen to accumulate at tumor sites and induce tumor apoptosis (Mi et al., 2019).
The targeting specificity of S. Typhimurium has been determined to be controlled via three receptors (Leschner et al., 2010).
- The aspartate chemoreceptor – initiating chemotaxis towards the tumor cylindroids (Leschner et al., 2010)
- The serine chemoreceptor – starting penetration (Leschner et al., 2010)
- The ribose/galactose receptor – directing the bacteria towards the necrotic region (Leschner et al., 2010).
In vitro studies have suggested that cancer cells produce compounds that specifically attract bacteria towards the necrotic regions of the tumor. The bacterial receptors involved in this process primarily function by controlling bacterial chemotaxis towards the necrotic regions of the tumor cylindroids (designed in the lab by constraining sphere-like structures between two parallel polymer surfaces, to mimic tumors for treatment testing). Thus, S. Typhimurium can be predicted to preferentially accumulate on areas because of the ribose/galactose receptors (Leschner et al., 2010).
The induction of tumor cell apoptosis (cell death) can be driven by multiple mechanisms such as bacterial toxin secretion, stimulation of immune response, and competition for nutrients (Mi et al., 2019). For instance, Salmonella can employ inhibitors to induce cell death via autophagic and apoptotic pathways (Mi et al., 2019). The bacterium significantly decreases the factors that negatively regulate autophagy by the AKT/mTOR signaling pathway, induces the production of proinflammatory molecules, and activates immune cells, changing the TME. Salmonella can also stimulate caspase-1, a molecule activated by immune cell structures called inflammasomes, to induce tumor cell apoptosis. This enzyme cleaves precursors of inflammatory cytokines (pro-IL-1 and pro-IL-18) to yield active IL-1 and IL-18 which have antitumor effects. Furthermore, being a facultative anaerobe, Salmonella can grow in both hypoxic and oxygen-rich tumor areas, allowing for deeper tumor penetration. Current research in Salmonella optimization is directed in large part towards discovering and developing various strains that can be viable for BT (Mi et al., 2019).
In the past decade, scientists from the International Institute of Anticancer Research reported increased efficacy against tumors with a sub-strain of S. Typhimurium A1-R (STAR1) (Uchugonova et al., 2012). This strain is auxotrophic for leucine and arginine, meaning that it cannot grow without external supplementation of leucine and arginine. Due to this, it could not mount a continuous infection in normal tissues (Uchugonova et al., 2012). Because of the nutritional milieu within the malignant tissue, however, STAR1 caused a persistent infection in tumors. In an experiment where STAR1 was labelled with green fluorescent protein (GFP) in order for its presence its presence to be tracked, the labeled bacteria were injected in the tail vein of PC-3 tumor-bearing nude mice. The bacteria was present lung, liver, spleen, and kidney tumors within 2-4 days after infection (Zhao et al., 2005 ). Over time, the bacterial number regressed significantly in normal tissue. By day 15, STAR1 was no longer seen in the normal tissues of the spleen, liver, kidney, and lung. The bacterial growth continued, however, in the PC-3 tumor tissue. In sum, Results showed that the A1 auxotrophs were able to selectively target the PC-3 tumor in vivo with essentially complete clearance from normal tissue (Zhao et al., 2005 ).
Following this study, detailed studies were conducted on this sub-strain using advanced imaging techniques like high-resolution multiphoton tomography to deduce the exact cancer-killing mechanism of bacteria (in live mice). Tumors were marked with fluorescent proteins, and both ‘in vivo’ and ‘in vitro’ observations were made (Uchugonova et al., 2012).
For in vitro experiments, the Lewis Lung Carcinoma (LLC) cell line expressing GFP in the nucleus and RFP in the cytoplasm was used. The cells were grown in RPMI 1640 medium in a 6-well tissue culture plate maintaining 105 cells/well density. STAR1 strain was grown on Luria broth (LB) medium and harvested in the late log phase. The bacteria were then diluted in a cell culture medium and added to cancer cells. Confocal microscopy was used to image dual-color LLC cells in vitro during bacteria treatment. The interaction between the cancer cells was observed at different points in time.
The LLC cell line used for the in vivo experiment had RFP in the cytoplasm and an H2B-GFP fusion gene for dual coloration and better visibility. The STAR1 strain (at late-log phase) was injected in tumor-bearing nude mice’s tail vein. An arc-shaped incision (skin-flap) was made in the skin to better image the tumor tissue. The skin flap could be repeatedly opened to image the cancer cells and closed with a 6-0 suture. The animals were anesthetized with a ketamine mixture of Ketaset & PromAce and Xylazine HCl (Uchugonova et al., 2012).
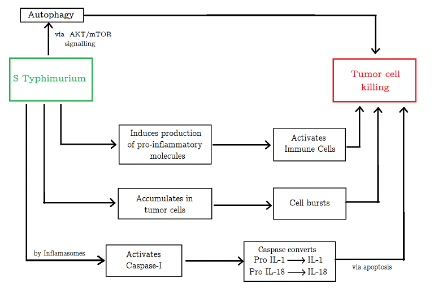
Figure 2: Tumor killing mechanisms employed by Salmonella. (Source: Image made by author)
Results of the aforementioned experiments explain the antitumor effects of the STAR1 sub-strain. After S. Typhimurium A1-R-GFP targeting, the membranes of LLC cells developed microblebs (minute rounded outgrowths on the surface of cells). These cells began to expand and lost their characteristic morphology. The nucleus of the cancer cells was destroyed in the following hours. The cells burst and lost their viability. The bacteria were able to leave capillaries minutes after IV injection. Within hours, the destruction of cancer cells started (Uchugonova et al., 2012).
This tumor regression was revealed by the RFP labeling of cancer cells (Uchugonova et al., 2012). Further research is needed to establish the underlying cancer-killing mechanism at a molecular level – i.e., which cancer cell proteins are impacted by bacterial infection.
Clostridium
Clostridium is a gram-positive anaerobic bacteria that has recently been assessed as a potential anti-cancer bacterial therapy (Feng et al., 2021). Due to their numerous peritrichous flagella, the Clostridium species C. novyi is highly mobile and oxygen-sensitive (Agrawal et al., 2004). It is therefore an ideal strain for BT as its spore infiltration into tumors is far superior to that of Bifidobacteria (anaerobic probiotics living in the intestine and stomach) (Feng et al., 2021). C. novyi quickly moves through the blood vessels and grows only in hypoxic zones (Agrawal et al., 2004). Arising from a small necrotic focus, it can rapidly spread throughout the tumor region (Agrawal et al., 2004).
However, like any other bacteria, spores of C. novyi also show high toxicity (Feng et al., 2021). The issue was addressed by Agrawal and colleagues in their experiment on the immunogenic effect of BT (2004). By removing the alpha-toxin gene from C. novyi, a new, harmless variant was obtained and named C. novyi-NT. Spores of C. novyi-NT were prepared, and mice were injected with 300 million spores suspended in 200 ul of PBS (Invitrogen) via the tail vein. Rabbits received 2 billion spores of C. novyi-NT suspended in 3 ml of PBS via the ear vein. (Agrawal et al., 2004)
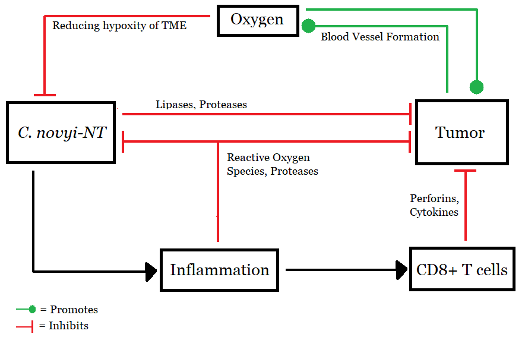
Figure 3: Working model of C. novyi-NT, (Source: Image made by author based on (Agrawal et al., 2004).
Cancer-causing cellular agents, CT26 (for mice) and RENCA (for rabbits), were implanted. Adoptive transfer (harvesting and diagnosing tumor cells ex vivo) of splenocytes and lymphocytes was done to separate CD4+ and CD8+ cells. Computed Tomography and Positron Emission Tomography confirmed moderately immunogenic tumors in syngeneic mice and no tumors in allogeneic mice. After 24 hours, central hemorrhagic necrosis was observed in tumors followed by neutrophil chemotaxis & activation, which lasted for another 48 hours. Three to four weeks into the experiment, 34% of the initial 47 mice were completely cured of cancer while in the remaining 66% small tumor regenerated from non-necrotic areas. 10 of the previous mice were further experimented upon, out of which 8 showed immunity to even larger CT26 dosages. Two potential explanations were hypothesized for this: either CT26 cells themselves triggered a weak immune response, or their mixture with C.novyi-NT provoked an immune response. When tested further, the latter was found to be true. Adoptive transfer techniques also demonstrated that CD8+ is preventing tumor growth and not CD4+. (Agrawal et al., 2004)
C. novyi-NT was also tested on rabbits by infecting their liver with VX2 tumor model (Agrawal et al., 2004). After just four days, inflammatory infiltrates replaced tumor cells. Gas pockets, a sign of Clostridial infection, were also found in some rabbits. This suggests C.novyi-NT caused the infection. (Inflammation occurs as a response to bacterial infection. The presence of inflammation supported the presence of bacteria, and suggested that the tumors had been digested by bacteria). From a sample size of 23 rabbits, seven were completely cured. As a test for immunity, three of those rabbits were injected with larger samples of VX2 after three, five, and eleven months of the experiment, respectively. None of them developed cancer. Apart from this, numerous other experiments emphasize the potential Clostridium (particularly C. novyi) holds for future cancer therapy (Agrawal et al., 2004).
E. Coli
E. coli is yet another bacteria used for mitigating tumor growth. This facultative anaerobe can be engineered to utilize the novel bystander killing strategy, which is instrumental in BT. In bystander killing, bacteria are induced to make a toxin that kills all the tumor cells around while keeping its own population unaffected. E. coli has been transfected with a gene that promotes the production of toxins. These toxins are responsible for killing tumor cells.
For in vivo conditions, existing vectors are not efficient enough and can only transduce less than 1% of targeted cells. It has been observed that the E. coli purine nucleoside phosphorylase (PNP) gene brings about cellular necrosis under conditions when 1 in 100 to 1 in 1000 cells express the gene product in vitro (Gadi et al., 2000).
Gadi and colleagues studied antitumor effects in the ovarian tumor model by transfecting cells with the E. coli PNP gene and further treating it with 9-(2- deoxy-erythro-pentofuranosyl)-6-methylpurine (MeP-dR) (2000). The E. coli PNP gene is controlled by a SV40 promoter. The PNP enzyme cleaves MeP-dR to liberate 6- methylpurine (MeP), a potent, membrane permeant and freely diffusible toxin. It was seen that 30 nmol MeP-dR was converted per milligram tumor cell extract per hour (or conversion units (CU)). After treatment with MeP-dR, this wipes out entire populations of tumor cells in vitro (Gadi et al., 2000).
For in vivo studies, Gadi and colleagues transfected human ovarian tumors with E. coli PNP (2000). These were excised five days after intraperitoneal (IP) implantation from the peritoneal cavities of mice in order to determine E. coli PNP enzymatic activity and the fraction of cells expressing the gene. E. coli PNP was expressed in approximately 0.1% of the tumor cells as judged by in situ hybridization. On treatment with MeP-dR, the expression of E. coli PNP at this level recorded a 30% increase in lifespan and a 49% reduction in tumor size compared to control tumors. These observations might lead one to infer that pronounced bystander killing by E. coli PNP is conferred in vivo (Gadi et al., 2000).
It is evident that vectors capable of transgene expression in as few as one in 1000 cells can produce substantial antitumor effects if the expression on a per-cell basis is very high. Therefore, minute fractions of tumor cells expressing the E. coli PNP gene can mediate measurable antitumor effects in a mouse model of human ovarian cancer (Gadi et al., 2000).
Apart from 6-methyl purine (MeP, used in the above experiment), other substrates for E. coli PNP are also available. Upon examining cytotoxicity data on various bases, Secrist III and colleagues came up with five such compounds, namely: 2-fluoroadenine (F-Ade), 6-methylpurine (MeP), 6-thioguanine, 2-amino-6- chloro-1-deazapurine, and 3-deazaguanine (1999). The first two are preferable because they are toxic to both proliferating and non-proliferating cells. The latter three act mainly on proliferating cell populations (1999). Furthermore, the nucleoside prodrug must be as non-toxic as possible to the body but a suitable substrate for E. coli PNP (Secrist III et al., 1999). All of these considerations must be kept in mind in selecting a purine analog that will convert into a toxic nucleoside metabolite when liberated into a transfected cell.
Another novel experiment, devised by Jiang and colleagues from Chonnam National University Medical School, Gwangju, demonstrates E. coli‘s integration with a pore-forming cytotoxic protein cytolysin A (ClyA) (2010). ClyA is a 34-kd protein toxin produced by E. Coli, Salmonella enterica serovar, Salmonella Typhi, and Salmonella Paratyphi A. It is transported to the surface of bacteria and is secreted without any post-translational modification. ClyA is cytotoxic toward cultured mammalian cells and induces macrophage apoptosis because of its pore-forming ability (Jiang et al., 2010). This differs from the former case, as PNP transcribed by E. coli had no toxicity of its own but required a substrate to produce toxic effects.
The setup is similar to the experiments mentioned earlier. One can engineer an E. coli strain (E. coli K-12) and use bioluminescence to detect cancer cell necrosis by specific proteins (Jiang et al., 2010). E. coli K-12 is non-pathogenic but has been engineered to express the genes encoding ClyA to kill tumor cells. It also possesses the Lux operon to help image infected tissues (Jiang et al., 2010). The bacterial luciferase (lux) gene cassette is a flavin-dependent monooxygenase gene cassette, remarkable for its distinctive feature in transforming chemical energy to photons of visible light. Further, it can synthesize and/or scavenge all of the substrate compounds required for its production of light, conferring self-sufficiency. Its bioluminescent signal in the transformed bacteria was detected using a cooled charge-coupled device camera (Xenogen-IVIS 100; Caliper, Hopkinton, MA).
However, very few engineered E. coli cells maintained the plasmid containing ClyA over time. To overcome this, Jiang and colleagues employed a “balanced-lethal host-vector system” using the gene encoding aspartate β -semialdehyde dehydrogenase (Asd) (2010). In order to retain plasmids in vivo, the chromosomal copy of Asd in the host strain was mutated and complemented by a vector carrying both the Asd gene and the Lux gene (Asd+pLux; pALux) or the Asd gene and the ClyA gene (Asd+pClyA; pAClyA) (Jiang et al., 2010).
To evaluate the tumor growth in primary and metastatic tumor models, E. coli K-12 was first transformed with pUC19 vector containing the Lux gene (pALux) (Jiang et al., 2010). Then bioluminescent signal in the transformed bacteria was detected using a cooled charge-coupled device camera (Xenogen-IVIS 100; Caliper, Hopkinton, MA). A clone was selected and
transformed with pAClyA. Western blot analysis (done using an anti-ClyA antibody) revealed a 34-kd protein corresponding to ClyA in the bacterial pellet and the culture medium, indicating that ClyA was successfully expressed. Bacteria producing Lux and ClyA can lyse blood cells and yield a clear bioluminescent signal in areas corresponding to the hemolysis. In the experiment performed by Sheng-Nan Jiang and colleagues from Chonnam National University Medical School, Gwangju, E. coli K-12 cells carrying Lux gene and ClyA gene were injected intravenously into immunocompetent mice bearing syngeneic tumors derived from CT26 colon carcinoma cells (2010). Lux gene expression was monitored, and it was observed that bioluminescent signals were only detected in the tumors of the mice. This shows that the genetically engineered bacteria have retained their ability to target the tumor cells, and that the bacterial treatment was tumor-specific (Jiang et al., 2010).
A single treatment with E. coli expressing ClyA markedly decreased tumor growth rates initially (9 days after treatment); however, the tumors grew after that (Jiang et al., 2010). With only radiotherapy, the tumor growth rates were retarded, but not the tumor sizes. E. Coli expressing ClyA along with radiation therapy resulted in significant shrinking of tumors and even complete disappearance of tumors in mice bearing CT26 colon cancer tumor. Furthermore, treating mice exhibiting metastatic tumor growth with E. Coli expressing ClyA markedly suppressed the tumor growth and prolonged the survival time in mice. This demonstrates the utility of genetically engineered E. coli in treating hypoxic tumor regions (Jiang et al., 2010).
Comparison and Combination Therapy
All three bacteria studied in this review possess distinct features and thus offer unique avenues of cancer treatment. E. coli can be genetically modified to release toxins that help destroy tumor cells. Clostridium deals with tumors by releasing toxins and elicits immune cells to act upon the tumors. Salmonella works by dysregulating the AKT/mTOR signaling pathway and stimulating enzymes to induce autophagy and apoptosis, respectively. Tumor cells develop microblebs (minute rounded outgrowths on the surface of cells) upon being infected by Salmonella and are destroyed as they burst and lose their viability.
Due to such enormous variations in their mechanisms, all three bacteria have some advantage over others. While Salmonella and Clostridium are lethal to cells, E. coli is made phagocytotic by genetic engineering, allowing administration via complex payloads. As facultative anaerobes, E. coli and Salmonella can thrive both in anaerobic and oxygen-rich conditions. This enables deeper tumor penetration than Clostridium – an anaerobic bacteria – which can only grow in oxygen-deficient areas of the tumor. Due to such oxygen sensitivity, its ability to eradicate tumors or tumor areas from oxygen-rich regions is severely limited. However, Clostridium is far more efficient than the other two bacteria in target specificity and evoking an immune response.
These differences become even more important if one considers using these bacteria in conjunction with other advanced techniques. These strategies fall under the umbrella term “combination therapies” (Wang et al., 2016). For example, one may use S. typhimurium to deliver herpes simplex virus thymidine kinase (HSV TK) with a beta-lactamase secretion signal to phosphorylate the prodrug ganciclovir (a nucleoside analogue) (Wang et al., 2016). Better tumor retardation and prolonged survival was observed with Salmonella/ganciclovir treatment as compared to BT alone. Combination therapy has significantly inhibited tumor growth (approximately 86.6–88.75%). Other prodrug converting enzymes such as cytosine deaminase (which converts fluorocytosine (5-FC) to 5-fluorouracil (5-FU)) have also been introduced to Salmonella vectors (Wang et al., 2016). Due to the high oxygen sensitivity of C. novyi-NT, its germination and spread are halted in well-oxygenated areas, with the bacteria forming a rim-like structure (Feng et al., 2021). Conventional chemotherapy and radiation therapy could be used to destroy the well-oxygenated cells in this rim. A combination of C. novyi-NT with these therapies might provide substantial antitumor effects (Agrawal et al., 2004).
Conclusion
Undoubtedly, administration of bacterial therapy holds promising results and can be a boon to cancer patients in the future. There are several arguments and experimental results supporting human implication of BT. It is effective against human xenografts, can develop in human wounds, and the obtained results are not species-specific (Agrawal et al., 2004). However, numerous issues like inadequate delivery methods to the tumor sites, targeting inefficiency, limited drug production, intrinsic bacterial toxicity, and genetic instability need to be addressed.
Most payloads delivered by tumor-targeting bacteria are toxic both to normal and tumor cells, mandating precise triggering and strict control in order for BT to be safe (Duong et al., 2019). Also, newly formed blood vessels in tumors are highly disorganized. They have incomplete endothelial linings and blind loops, resulting in poor blood flow and inefficient delivery of nutrients and oxygen to regions within the tumor tissues, forming areas of anoxia (Jiang et al., 2010). These multiple hypoxic regions within the tumor make payload delivering efficiency even worse. Moreover, the ability of human tumors to evade potential immunogenic responses is a further challenge in human implication of bacterial treatment (especially the ones that function by initiating immune responses) (Agrawal et al., 2004).
Apart from these complications, a few experimental constraints also cannot be ignored. The sample sizes for tumor testing in the studies mentioned throughout this article were very small and minutely diverse, both geographically and genetically (Sieow et al., 2021). BT’s combined effects with other humanoid conditions (pathogenic deficiency diseases, genetic disorders, etc.) are yet to be studied. Thus, enormous amount of research is needed and is being done in the field of Bacterial Cancer Treatment (Sieow et al., 2021). New delivery methods, including viral or other vectors, are being developed for selective tumor targeting (Sieow et al., 2021). Advances in microbiology like improved understanding of quorum sensing and development of gene circuit modification techniques are being devised and tested every passing second (Sieow et al., 2021). Scientists have created a whole arsenal of therapeutic payloads to be delivered by engineered bacteria, each conditioned for a specific strategy (Sieow et al., 2021). In conclusion, it is pretty realistic to say that the boundaries limiting applications of BT can indeed be broken, and with further advancements, the human application of BT will surely see light of the day.
References
Cancer. (2022, February 3). World Health Organization. https://www.who.int/news-room/fact-sheets/detail/cancer
Duong, M. T.-Q., Qin, Y., You, S.-H., & Min, J.-J. (2019). Bacteria-cancer interactions: bacteria-based cancer therapy. Experimental & Molecular Medicine. https://doi.org/10.1038/s12276-019-0297-0
Coley, W. B. (1936). Bullet Wounds of the Abdomen. The British Medical Journal, 1. https://www.jstor.org/stable/25352438
The Toxin Treatment Of Sarcoma. (1908). The British Medical Journal, 1, 1064–1065. https://www.jstor.org/stable/25277459
Mi, Z., Feng, Z. C., Li, C., Yang, X., Ma, M. T., & Rong, P. F. (2019). Salmonella-Mediated Cancer Therapy: An Innovative Therapeutic Strategy. Journal of Cancer, 10(20), 4765–4776. https://doi.org/10.7150/jca.32650
Leschner, S., & Weiss, S. (2010). Salmonella-allies in the fight against cancer. Journal of molecular medicine (Berlin, Germany), 88(8), 763–773. https://sci-hub.ee/10.1007/s00109-010-0636-z
Uchugonova, A., Zhao, M., Zhang, Y., Weinigel, M., Konig, K., & Hoffman, R. M. (2012). Cancer-cell Killing by Engineered Salmonella Imaged by Multiphoton Tomography in Live Mice. Anticancer Research, 32(10), 4331–4337. https://ar.iiarjournals.org/content/32/10/4331.full
Zhao, M., Yang, M., Li, X.-M., Jiang, P., Baranov, E., Li, S., Xu, M., Penman, S., & Hoffman, R. M. (2005). Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. Proceedings of the National Academy of Sciences, 102(3), 755–760. https://doi.org/10.1073/pnas.0408422102
Agrawal, N., Bettegowda, C., Cheong, I., Geschwind, J.-F., Drake, C. G., Hipkiss, E. L., Tatsumi, M., Dang, L. H., Diaz Jr., L. A., Pomper, M., Abusedera, M., Wahl, R. L., Kinzler, K. W., Zhou, S., Huso, D. L., & Vogelstein, B. (2004). Bacteriolytic Therapy Can Generate a Potent Immune Response against Experimental Tumors. Proceedings of the National Academy of Sciences of the United States of America, 101, 15172–15177. https://www.jstor.org/stable/3373595
Feng, X., He, P., Zeng, C., Li, Y., Das, S.K., Li, B. … Du, Y. (2021). Novel insights into the role of Clostridium novyi-NT related combination bacteriolytic therapy in solid tumors (Review). Oncology Letters, 21, 110. https://doi.org/10.3892/ol.2020.12371
(Feng et al., 2021)
Gadi, V. K., Alexander, S. D., Kudlow, J. E., Allan, P., Parker, W. B., & Sorscher, E. J. (2000). In vivo sensitization of ovarian tumors to chemotherapy by expression of E. coli purine nucleoside phosphorylase in a small fraction of cells. Gene Therapy, 7, 1738–1743. https://doi.org/10.1038/sj.gt.3301286
Secrist III, J. A., Parker, W. B., Allan, P. W., Bennett Jr., L. L., Waud, W. R., Truss, J. W., Fowler, A. T., Montgomery, J. A., Ealick, S. E., Wells, A. H., Gillespie, G. Y., Gadi, V. K., & Sorscher, E. J. (1999). : Gene Therapy of Cancer: Activation of Nucleoside Prodrugs with E. coli Purine Nucleoside Phosphorylase. Nucleosides and Nucleotides, 18(4–5), 745–757. https://doi.org/10.1080/15257779908041562
Jiang, S.-N., Phan, T. X., Nam, T.-K., Nguyen, V. H., Kim, H.-S., Bom, H.-S., Choy, H. E., Hong, Y., & Min, J.-J. (2010). Inhibition of Tumor Growth and Metastasis by a Combination of Escherichia coli–mediated Cytolytic Therapy and Radiotherapy. Molecular Therapy, 18(3), 635–642. https://doi.org/10.1038/mt.2009.295
Wang, C.-Z., Kazmierczak, R. A., & Eisenstark, A. (2016). Strains, Mechanism, and Perspective: Salmonella-Based Cancer Therapy. International Journal of Microbiology. https://doi.org/10.1155/2016/5678702
Sieow, B. F.-L., Wun, K. S., Yong, W. P., Hwang, I. Y., & Chang, M. W. (2021). Tweak to Treat: Reprograming Bacteria for Cancer Treatment. Trends in Cancer, 7(5), 447–464. https://doi.org/10.1016/j.trecan.2020.11.004
Related Posts
SARS-CoV-2 is not the only virus living among us
Figure 1: The human gut is home to a rich...
Read MoreStaphylococcus epidermidis capable of 6-HAP production reduce host UV-induced tumors
Figure 1: The human microbiome is the collection of microorganisms...
Read MoreExploring Possibilities for the Early Diagnosis of Alzheimer’s Disease
Figure 1: Extracellular deposits of amyloid-beta proteins as seen in...
Read MoreModeling Maximum Running Speed in Animals
Figure: Two cheetahs lie side by side. As the fastest...
Read MoreThe Gut May Be The Door to Effective Depression Treatment
First Author: Jillian Troth1 Co-Authors [Alphabetical Order]: Roxanna Attar2, Caroline...
Read MoreBad dreams? According to new studies, COVID-19 could be to blame
Figure 1: A sleeping child. When we sleep, we dream,...
Read MoreMerrick and Venkatesh Jha