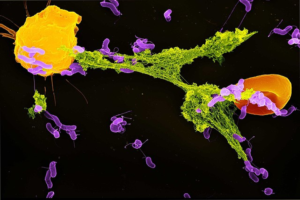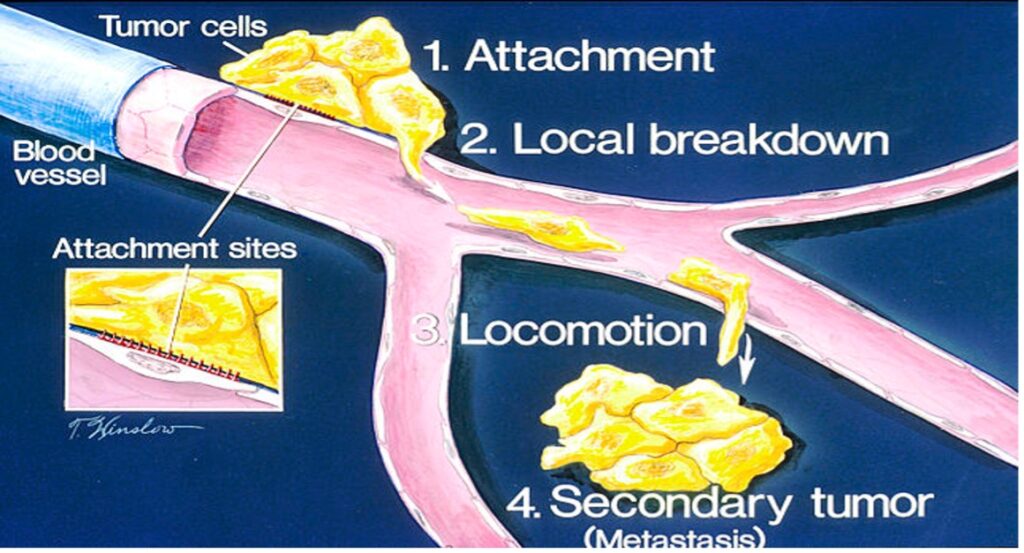
Figure 1: The start of metastasis – the movement of tumor cells from the initial site of tumor growth to other regions of the body
Source: Wikimedia Commons
Metastasis is an outcome that often turns treatable cancer into a fatal disease. It occurs when circulating tumor cells acquire the ability to penetrate the walls of lymphatic or blood vessels; they are then able to circulate through the bloodstream to other sites and tissues in the body (Maheswaran & Haber, 2010). Metastasis often leads to deteriorating condition and a bleaker prognosis. Despite the gravity of the problem, no conclusive prediction techniques yet exist to map out the progress of metastatic cancers in order to determine better interventions. Tracking metastatic cells is especially hard given the complex transport mechanisms within the body that shuttle tumor cells around body tissues.
Most studies on metastasis rely on a small number of experimental models, making it difficult to extrapolate findings to genetically diverse human tumors (Barretina et al., 2012). More recently, however, the synthesis of a definitive method to map out metastasis on a cell-to-cell basis was developed. The system was pioneered by researchers from the Broad Institute of MIT as well as researchers from Harvard University.
The researchers first conducted a pilot study involving breast cancer cell. They engineered cells to express a 26-nucleotide marker “barcode” with luciferase, an enzyme that produces bioluminescent signals, to allow for in vivo imaging and cell sorting assays. The barcoded cell lines were then injected into lab rats, and the researchers tracked the ability of tumor cells to exit circulation and undergo expansion in distant organs. After five weeks, bioluminescence revealed metastatic lesions all around the body, which implied that there was metastatic spread of the tumor cells. Bulk genetic codes were then identified by RNA sequencing and independently validated by quantitative PCR (Jin et al., 2020).
After demonstrating feasibility in breast cancer, Jin et al. then decided to scale up their study with cell lines form diverse lineages. To reflect cancer cell diversity in the data set, they varied cell numbers, mouse age, and cohort size. The method discussed in the previous paragraph was applied to 503 cell lines consisting of 21 lineages and the statistical results were used to generate a first generation Metastasis map (Jin et al., 2020).
Data analysis found statistical correlations between tumor lineage, the site where the cell line was derived, and patient age. From the outcomes, the team determined that social factors, such as gender and ethnicity did not directly affect metastatic potential. Intracardiac administration; direct injection of the metastatic cells into the heart for a systemic spread via the blood stream, proved to be more potent in facilitation of metastasis compared to subcutaneous route; injection into the dermis. In the experiment, 197 cell lines were recovered for the intracardiac route with only 42 for the subcutaneous route. The incongruities were attributed to the local competition for nutrients and other microenvironment factors in the subcutaneous setting whereas the spatial separation of tumor cells administered via the intracardiac route minimized such competition (Jin et al., 2020).
Moreover, metastatic potential was higher in certain tumor types, most notably melanoma and pancreatic cancer. This makes sense – these cancers have been observed have high rates of metastisis in the clinical setting (Budczies et al., 2014). Brain tumors were generally non metastatic. This is likely due to the presence of the blood brain barrier, which restricts movement of molecules and cells in and out of the blood vessels of the brain. The positive association between metastatic potential and increased patient age was unexpected, and its basis remains to be determined. The most probable explanation would be the decline in immunological strength with age such as thymic atrophy which diminishes the supply and competence of T cells (Montecino-Rodriguez et al., 2013).
According to the researchers, their pivotal finding was that of extensive variation in individual metastatic cell lineages of the same cancer. This discreteness in cell lines is especially indispensable as it potentiates the ability to search for specific associations between metastasis and genomic features encoded in the tumor (Jin et al., 2020). Basing on these findings, metastasis maps may soon be integrated into the long-term evaluation of cancer progression in chronic cases, providing an incredibly effective way to identify and treat metastatic tumours.
References
Barretina, J., Caponigro, G., Stransky, N., Venkatesan, K., Margolin, A. A., Kim, S., Wilson, C. J., Lehár, J., Kryukov, G. V., Sonkin, D., Reddy, A., Liu, M., Murray, L., Berger, M. F., Monahan, J. E., Morais, P., Meltzer, J., Korejwa, A., Jané-Valbuena, J., … Garraway, L. A. (2012). The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature, 483(7391), 603–607. https://doi.org/10.1038/nature11003
Budczies, J., von Winterfeld, M., Klauschen, F., Bockmayr, M., Lennerz, J. K., Denkert, C., Wolf, T., Warth, A., Dietel, M., Anagnostopoulos, I., Weichert, W., Wittschieber, D., & Stenzinger, A. (2014). The landscape of metastatic progression patterns across major human cancers. Oncotarget, 6(1), 570–583.
Jin, X., Demere, Z., Nair, K., Ali, A., Ferraro, G. B., Natoli, T., Deik, A., Petronio, L., Tang, A. A., Zhu, C., Wang, L., Rosenberg, D., Mangena, V., Roth, J., Chung, K., Jain, R. K., Clish, C. B., Vander Heiden, M. G., & Golub, T. R. (2020). A metastasis map of human cancer cell lines. Nature, 588(7837), 331–336. https://doi.org/10.1038/s41586-020-2969-2
Maheswaran, S., & Haber, D. A. (2010). Circulating tumor cells: A window into cancer biology and metastasis. Current Opinion in Genetics & Development, 20(1), 96–99. https://doi.org/10.1016/j.gde.2009.12.002
Montecino-Rodriguez, E., Berent-Maoz, B., & Dorshkind, K. (2013). Causes, consequences, and reversal of immune system aging. The Journal of Clinical Investigation, 123(3), 958–965. https://doi.org/10.1172/JCI64096
Related Posts
Neutrophils and their NETs
Figure: A neutrophil (yellow) ejecting a NET (green) to capture...
Read MoreThe E.C.H.O. Initiative: Encouraging Cardiovascular Health Through Outreach
This publication is in proud partnership with Project UNITY’s Catalyst Academy 2023...
Read MoreWhitney Nicanor Mabwi